Subtotal parathyroidectomy for secondary renal hyperparathyroidism: a 20-year surgical outcome study
- PMID: 27233241
- PMCID: PMC5086343
- DOI: 10.1007/s00423-016-1447-7
Subtotal parathyroidectomy for secondary renal hyperparathyroidism: a 20-year surgical outcome study
Abstract
Aim: The aim of this study was to evaluate the outcomes of surgery for patients with secondary renal hyperparathyroidism (rHPT).
Methods: This is a retrospective cohort study. Our institutional database was searched for eligible patients treated in 1995-2014. The inclusion criterion was initial parathyroidectomy for rHPT. Clinical and follow-up data were analyzed to estimate the cure rate (primary outcome), and morbidity (secondary outcome).
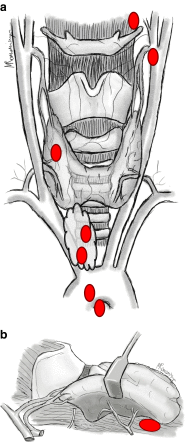
Results: The study group comprised 297 patients (154 females, age 44.5 ± 13.7 years, follow-up 24.6 ± 10.5 months), including 268 (90.2 %) patients who had underwent subtotal parathyroidectomy, and 29 (9.8 %) who had had incomplete parathyroidectomy. Intraoperative iPTH assay was utilized in 207 (69.7 %) explorations. Persistent rHPT occurred in 12/268 (4.5 %) patients after subtotal parathyroidectomy and 5/29 (17.2 %) subjects after incomplete parathyroidectomy (p = 0.005). The patients operated on with intraoperative iPTH assay had a higher cure rate than non-monitored individuals, 201/207 (97.1 %) vs. 79/90 (87.8 %), respectively (p = 0.001). In-hospital mortality occurred in 1/297 (0.3 %) patient. The hungry bone syndrome occurred in 84/268 (31.3 %) patients after subtotal parathyroidectomy and 2/29 (6.9 %) subjects after incomplete parathyroidectomy (p = 0.006). Transient recurrent laryngeal nerve paresis occurred in 14/594 (2.4 %) and permanent in 5/594 (0.8 %) nerves at risk.
Conclusions: Subtotal parathyroidectomy is a safe and efficacious treatment for patients with rHPT. Utilization of intraoperative iPTH assay can guide surgical exploration and improve the cure rate.
Keywords: Intraoperative iPTH assay; Secondary renal hyperparathyroidism; Subtotal parathyroidectomy.
Conflict of interest statement
Compliance with ethical standards Ethics approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent For this type of study, formal consent is not required.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

