A cognitive prosthesis for memory facilitation by closed-loop functional ensemble stimulation of hippocampal neurons in primate brain
- PMID: 27233622
- PMCID: PMC5633045
- DOI: 10.1016/j.expneurol.2016.05.031
A cognitive prosthesis for memory facilitation by closed-loop functional ensemble stimulation of hippocampal neurons in primate brain
Abstract
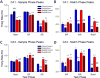
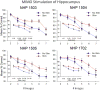
Very productive collaborative investigations characterized how multineuron hippocampal ensembles recorded in nonhuman primates (NHPs) encode short-term memory necessary for successful performance in a delayed match to sample (DMS) task and utilized that information to devise a unique nonlinear multi-input multi-output (MIMO) memory prosthesis device to enhance short-term memory in real-time during task performance. Investigations have characterized how the hippocampus in primate brain encodes information in a multi-item, rule-controlled, delayed match to sample (DMS) task. The MIMO model was applied via closed loop feedback micro-current stimulation during the task via conformal electrode arrays and enhanced performance of the complex memory requirements. These findings clearly indicate detection of a means by which the hippocampus encodes information and transmits this information to other brain regions involved in memory processing. By employing the nonlinear dynamic multi-input/multi-output (MIMO) model, developed and adapted to hippocampal neural ensemble firing patterns derived from simultaneous recorded multi-neuron CA1 and CA3 activity, it was possible to extract information encoded in the Sample phase of DMS trials that was necessary for successful performance in the subsequent Match phase of the task. The extension of this MIMO model to online delivery of electrical stimulation patterns to the same recording loci that exhibited successful CA1 firing in the DMS Sample Phase provided the means to increase task performance on a trial-by-trial basis. Increased utility of the MIMO model as a memory prosthesis was exhibited by the demonstration of cumulative increases in DMS task performance with repeated MIMO stimulation over many sessions. These results, reported below in this article, provide the necessary demonstrations to further the feasibility of the MIMO model as a memory prosthesis to recover and/or enhance encoding of cognitive information in humans with memory disruptions resulting from brain injury, disease or aging.
Keywords: Closed-loop; Electrical stimulation; Ensemble; Hippocampus; Memory; Neural prosthesis; Nonlinear model.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
A nonlinear model for hippocampal cognitive prosthesis: memory facilitation by hippocampal ensemble stimulation.IEEE Trans Neural Syst Rehabil Eng. 2012 Mar;20(2):184-97. doi: 10.1109/TNSRE.2012.2189163. IEEE Trans Neural Syst Rehabil Eng. 2012. PMID: 22438334 Free PMC article.
-
A cortical neural prosthesis for restoring and enhancing memory.J Neural Eng. 2011 Aug;8(4):046017. doi: 10.1088/1741-2560/8/4/046017. Epub 2011 Jun 15. J Neural Eng. 2011. PMID: 21677369 Free PMC article.
-
Facilitation of memory encoding in primate hippocampus by a neuroprosthesis that promotes task-specific neural firing.J Neural Eng. 2013 Dec;10(6):066013. doi: 10.1088/1741-2560/10/6/066013. Epub 2013 Nov 12. J Neural Eng. 2013. PMID: 24216292 Free PMC article.
-
Voltage Imaging in the Study of Hippocampal Circuit Function and Plasticity.Adv Exp Med Biol. 2015;859:197-211. doi: 10.1007/978-3-319-17641-3_8. Adv Exp Med Biol. 2015. PMID: 26238054 Review.
-
The role of hippocampal subregions in memory for stimulus associations.Behav Brain Res. 2010 Dec 31;215(2):275-91. doi: 10.1016/j.bbr.2010.07.006. Epub 2010 Jul 13. Behav Brain Res. 2010. PMID: 20633579 Review.
Cited by
-
Neuroprosthetics: from sensorimotor to cognitive disorders.Commun Biol. 2023 Jan 6;6(1):14. doi: 10.1038/s42003-022-04390-w. Commun Biol. 2023. PMID: 36609559 Free PMC article. Review.
-
Injecting Information into the Mammalian Cortex: Progress, Challenges, and Promise.Neuroscientist. 2021 Apr;27(2):129-142. doi: 10.1177/1073858420936253. Epub 2020 Jul 10. Neuroscientist. 2021. PMID: 32648527 Free PMC article. Review.
-
Brain-Machine Interfaces: Powerful Tools for Clinical Treatment and Neuroscientific Investigations.Neuroscientist. 2019 Apr;25(2):139-154. doi: 10.1177/1073858418775355. Epub 2018 May 17. Neuroscientist. 2019. PMID: 29772957 Free PMC article. Review.
-
Individually customized transcranial temporal interference stimulation for focused modulation of deep brain structures: a simulation study with different head models.Sci Rep. 2020 Jul 16;10(1):11730. doi: 10.1038/s41598-020-68660-5. Sci Rep. 2020. PMID: 32678264 Free PMC article.
-
Non-invasive brain stimulation and neuroenhancement.Clin Neurophysiol Pract. 2022 May 25;7:146-165. doi: 10.1016/j.cnp.2022.05.002. eCollection 2022. Clin Neurophysiol Pract. 2022. PMID: 35734582 Free PMC article. Review.
References
-
- Berger TW, Glanzman DL. Toward Replacement Parts for the Brain. MIT Press; Cambridge, MA: 2005.
-
- Berger TW, Ahuja A, Courellis SH, Deadwyler SA, Erinjippurath G, Gerhardt GA, Gholmieh G, Granacki JJ, Hampson R, Hsaio MC, LaCoss J, Marmarelis VZ, Nasiatka P, Srinivasan V, Song D, Tanguay AR, Wills J. Restoring lost cognitive function. IEEE Eng Med. Biol. Mag. 2005;24:30–44. - PubMed
-
- Chapin JK. Using multi-neuron population recordings for neural prosthetics. Nat. Neurosci. 2004;7:452–455. - PubMed
-
- Davachi L. Item, context and relational episodic encoding in humans. Curr. Opin. Neurobiol. 2006;16:693–700. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

