Activation of toll-like receptor signaling pathways leading to nitric oxide-mediated antiviral responses
- PMID: 27233799
- PMCID: PMC7087267
- DOI: 10.1007/s00705-016-2904-x
Activation of toll-like receptor signaling pathways leading to nitric oxide-mediated antiviral responses
Abstract
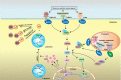
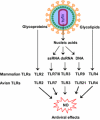
Toll-like receptors (TLRs), well-characterized pattern-recognizing receptors of the innate arm of the immune system, are vital in detecting pathogen-associated molecular patterns (PAMPs). The TLR-PAMP interaction initiates an intracellular signaling cascade, predominantly culminating in upregulation of antiviral components, including inducible nitric oxide synthase (iNOS). After activation, various TLR pathways can promote iNOS production via the myeloid differentiation primary response-88 (MyD-88) adapter protein. Subsequently, iNOS facilitates production of nitric oxide (NO), a highly reactive and potent antiviral molecule that can inhibit replication of RNA and DNA viruses. Furthermore, NO can diffuse freely across cell membranes and elicit antiviral mechanisms in various ways, including direct and indirect damage to viral genomes. This review emphasizes current knowledge of NO-mediated antiviral responses elicited after activation of TLR signaling pathways.
Conflict of interest statement
Mohamed Sarjoon Abdul-Cader declares that he has no conflict of interest. Aruna Amarasinghe declares that he has no conflict of interest. Mohamed Faizal Abdul-Careem declares that he has no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

