Biosynthesis of oxidized lipid mediators via lipoprotein-associated phospholipase A2 hydrolysis of extracellular cardiolipin induces endothelial toxicity
- PMID: 27233995
- PMCID: PMC5142456
- DOI: 10.1152/ajplung.00038.2016
Biosynthesis of oxidized lipid mediators via lipoprotein-associated phospholipase A2 hydrolysis of extracellular cardiolipin induces endothelial toxicity
Abstract
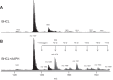
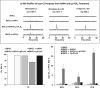
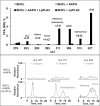
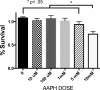
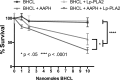
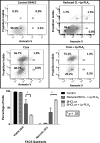
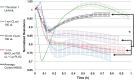
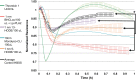
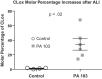
We (66) have previously described an NSAID-insensitive intramitochondrial biosynthetic pathway involving oxidation of the polyunsaturated mitochondrial phospholipid, cardiolipin (CL), followed by hydrolysis [by calcium-independent mitochondrial calcium-independent phospholipase A2-γ (iPLA2γ)] of oxidized CL (CLox), leading to the formation of lysoCL and oxygenated octadecadienoic metabolites. We now describe a model system utilizing oxidative lipidomics/mass spectrometry and bioassays on cultured bovine pulmonary artery endothelial cells (BPAECs) to assess the impact of CLox that we show, in vivo, can be released to the extracellular space and may be hydrolyzed by lipoprotein-associated PLA2 (Lp-PLA2). Chemically oxidized liposomes containing bovine heart CL produced multiple oxygenated species. Addition of Lp-PLA2 hydrolyzed CLox and produced (oxygenated) monolysoCL and dilysoCL and oxidized octadecadienoic metabolites including 9- and 13-hydroxyoctadecadienoic (HODE) acids. CLox caused BPAEC necrosis that was exacerbated by Lp-PLA2 Lower doses of nonlethal CLox increased permeability of BPAEC monolayers. This effect was exacerbated by Lp-PLA2 and partially mimicked by authentic monolysoCL or 9- or 13-HODE. Control mice plasma contained virtually no detectable CLox; in contrast, 4 h after Pseudomonas aeruginosa (P. aeruginosa) infection, 34 ± 8 mol% (n = 6; P < 0.02) of circulating CL was oxidized. In addition, molar percentage of monolysoCL increased twofold after P. aeruginosa in a subgroup analyzed for these changes. Collectively, these studies suggest an important role for 1) oxidation of CL in proinflammatory environments and 2) possible hydrolysis of CLox in extracellular spaces producing lysoCL and oxidized octadecadienoic acid metabolites that may lead to impairment of pulmonary endothelial barrier function and necrosis.
Keywords: cardiolipin; lineolic acid; lipoprotein-associated phospholipase A2; octadecadienoic acid; pulmonary endothelium.
Copyright © 2016 the American Physiological Society.
Figures



Similar articles
-
The phospholipase iPLA2γ is a major mediator releasing oxidized aliphatic chains from cardiolipin, integrating mitochondrial bioenergetics and signaling.J Biol Chem. 2017 Jun 23;292(25):10672-10684. doi: 10.1074/jbc.M117.783068. Epub 2017 Apr 25. J Biol Chem. 2017. PMID: 28442572 Free PMC article.
-
Disentangling oxidation/hydrolysis reactions of brain mitochondrial cardiolipins in pathogenesis of traumatic injury.JCI Insight. 2018 Nov 2;3(21):e97677. doi: 10.1172/jci.insight.97677. JCI Insight. 2018. PMID: 30385716 Free PMC article.
-
Radiation-induced lipoprotein-associated phospholipase A2 increases lysophosphatidylcholine and induces endothelial cell damage.Toxicology. 2021 Jun 30;458:152841. doi: 10.1016/j.tox.2021.152841. Epub 2021 Jun 30. Toxicology. 2021. PMID: 34216699
-
[Does Lp-PLA2 determination help predict atherosclerosis and cardiocerebrovascular disease?].Acta Med Croatica. 2010 Oct;64(4):237-45. Acta Med Croatica. 2010. PMID: 21688606 Review. Croatian.
-
The role of lipoprotein-associated phospholipase A2 as a marker for atherosclerosis.Curr Atheroscler Rep. 2007 Aug;9(2):97-103. doi: 10.1007/s11883-007-0004-9. Curr Atheroscler Rep. 2007. PMID: 17877917 Review.
Cited by
-
Outer Membrane Lipid Secretion and the Innate Immune Response to Gram-Negative Bacteria.Infect Immun. 2020 Jun 22;88(7):e00920-19. doi: 10.1128/IAI.00920-19. Print 2020 Jun 22. Infect Immun. 2020. PMID: 32253250 Free PMC article. Review.
-
Mitophagy in atherosclerosis: from mechanism to therapy.Front Immunol. 2023 May 16;14:1165507. doi: 10.3389/fimmu.2023.1165507. eCollection 2023. Front Immunol. 2023. PMID: 37261351 Free PMC article. Review.
-
The phospholipase iPLA2γ is a major mediator releasing oxidized aliphatic chains from cardiolipin, integrating mitochondrial bioenergetics and signaling.J Biol Chem. 2017 Jun 23;292(25):10672-10684. doi: 10.1074/jbc.M117.783068. Epub 2017 Apr 25. J Biol Chem. 2017. PMID: 28442572 Free PMC article.
-
Mitochondrial dysfunction in vascular endothelial cells and its role in atherosclerosis.Front Physiol. 2022 Dec 20;13:1084604. doi: 10.3389/fphys.2022.1084604. eCollection 2022. Front Physiol. 2022. PMID: 36605901 Free PMC article. Review.
-
Ischemia In Vivo Induces Cardiolipin Oxidation in Rat Kidney Mitochondria.Biology (Basel). 2022 Mar 31;11(4):541. doi: 10.3390/biology11040541. Biology (Basel). 2022. PMID: 35453739 Free PMC article.
References
-
- Aoki H, Hisada T, Ishizuka T, Utsugi M, Kawata T, Shimizu Y, Okajima F, Dobashi K, Mori M. Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochem Biophys Res Commun 367: 509–515, 2008. - PubMed
-
- Ariel A, Serhan CN. Resolvins and protectins in the termination program of acute inflammation. Trends Immunol 28: 176–183, 2007. - PubMed
-
- Arslan F, de Kleijn DP, Pasterkamp G. Innate immune signaling in cardiac ischemia. Nat Rev Cardiol 8: 292–300, 2011. - PubMed
-
- Balasubramanian K, Maeda A, Lee JS, Mohammadyani D, Dar HH, Jiang JF, St Croix CM, Watkins S, Tyurin VA, Tyurina YY, Kloditz K, Polimova A, Kapralova VI, Xiong Z, Ray P, Klein-Seetharaman J, Mallampalli RK, Bayir H, Fadeel B, Kagan VE. Dichotomous roles for externalized cardiolipin in extracellular signaling: Promotion of phagocytosis and attenuation of innate immunity. Sci Signal 8: ra95, 2015. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

