Histone deacetylation contributes to low extracellular superoxide dismutase expression in human idiopathic pulmonary arterial hypertension
- PMID: 27233998
- PMCID: PMC4967185
- DOI: 10.1152/ajplung.00263.2015
Histone deacetylation contributes to low extracellular superoxide dismutase expression in human idiopathic pulmonary arterial hypertension
Abstract
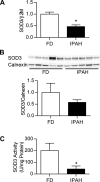
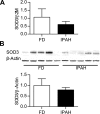
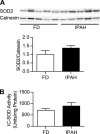
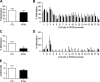
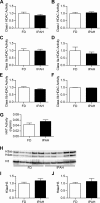
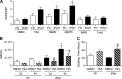
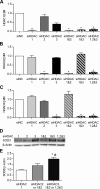
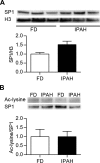
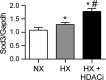
Epigenetic mechanisms, including DNA methylation and histone acetylation, regulate gene expression in idiopathic pulmonary arterial hypertension (IPAH). These mechanisms can modulate expression of extracellular superoxide dismutase (SOD3 or EC-SOD), a key vascular antioxidant enzyme, and loss of vascular SOD3 worsens outcomes in animal models of pulmonary arterial hypertension. We hypothesized that SOD3 gene expression is decreased in patients with IPAH due to aberrant DNA methylation and/or histone deacetylation. We used lung tissue and pulmonary artery smooth muscle cells (PASMC) from subjects with IPAH at transplantation and from failed donors (FD). Lung SOD3 mRNA expression and activity was decreased in IPAH vs. FD. In contrast, mitochondrial SOD (Mn-SOD or SOD2) protein expression was unchanged and intracellular SOD activity was unchanged. Using bisulfite sequencing in genomic lung or PASMC DNA, we found the methylation status of the SOD3 promoter was similar between FD and IPAH. Furthermore, treatment with 5-aza-2'-deoxycytidine did not increase PASMC SOD3 mRNA, suggesting DNA methylation was not responsible for PASMC SOD3 expression. Though total histone deacetylase (HDAC) activity, histone acetyltransferase (HAT) activity, acetylated histones, and acetylated SP1 were similar between IPAH and FD, treatment with two selective class I HDAC inhibitors increased SOD3 only in IPAH PASMC. Class I HDAC3 siRNA also increased SOD3 expression. Trichostatin A, a pan-HDAC inhibitor, decreased proliferation in IPAH, but not in FD PASMC. These data indicate that histone deacetylation, specifically via class I HDAC3, decreases SOD3 expression in PASMC and HDAC inhibitors may protect IPAH in part by increasing PASMC SOD3 expression.
Keywords: DNA methylation; extracellular superoxide dismutase; histone deacetylation; idiopathic pulmonary arterial hypertension.
Copyright © 2016 the American Physiological Society.
Figures
Similar articles
-
Regulation of Oxidative Stress in Pulmonary Artery Endothelium. Modulation of Extracellular Superoxide Dismutase and NOX4 Expression Using Histone Deacetylase Class I Inhibitors.Am J Respir Cell Mol Biol. 2015 Oct;53(4):513-24. doi: 10.1165/rcmb.2014-0260OC. Am J Respir Cell Mol Biol. 2015. PMID: 25749103 Free PMC article.
-
Pulmonary artery smooth muscle cell proliferation and migration in fetal lambs acclimatized to high-altitude long-term hypoxia: role of histone acetylation.Am J Physiol Lung Cell Mol Physiol. 2012 Dec 1;303(11):L1001-10. doi: 10.1152/ajplung.00092.2012. Epub 2012 Oct 5. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 23043075 Free PMC article.
-
Epigenetic regulation of EC-SOD expression in aging lung fibroblasts: Role of histone acetylation.Free Radic Biol Med. 2017 Nov;112:212-223. doi: 10.1016/j.freeradbiomed.2017.07.028. Epub 2017 Jul 27. Free Radic Biol Med. 2017. PMID: 28757400
-
Acquired Mitochondrial Abnormalities, Including Epigenetic Inhibition of Superoxide Dismutase 2, in Pulmonary Hypertension and Cancer: Therapeutic Implications.Adv Exp Med Biol. 2016;903:29-53. doi: 10.1007/978-1-4899-7678-9_3. Adv Exp Med Biol. 2016. PMID: 27343087 Review.
-
Calcium-Sensing Receptor Regulates Cytosolic [Ca 2+ ] and Plays a Major Role in the Development of Pulmonary Hypertension.Front Physiol. 2016 Nov 4;7:517. doi: 10.3389/fphys.2016.00517. eCollection 2016. Front Physiol. 2016. PMID: 27867361 Free PMC article. Review.
Cited by
-
The role of HDAC3 and its inhibitors in regulation of oxidative stress and chronic diseases.Cell Death Discov. 2023 Apr 18;9(1):131. doi: 10.1038/s41420-023-01399-w. Cell Death Discov. 2023. PMID: 37072432 Free PMC article. Review.
-
Citrate: a key signalling molecule and therapeutic target for bone remodeling disorder.Front Endocrinol (Lausanne). 2025 Jan 16;15:1512398. doi: 10.3389/fendo.2024.1512398. eCollection 2024. Front Endocrinol (Lausanne). 2025. PMID: 39886032 Free PMC article. Review.
-
Update on novel targets and potential treatment avenues in pulmonary hypertension.Am J Physiol Lung Cell Mol Physiol. 2016 Nov 1;311(5):L811-L831. doi: 10.1152/ajplung.00302.2016. Epub 2016 Sep 2. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 27591245 Free PMC article. Review.
-
Extracellular Superoxide Dismutase Regulates Early Vascular Hyaluronan Remodeling in Hypoxic Pulmonary Hypertension.Sci Rep. 2020 Jan 14;10(1):280. doi: 10.1038/s41598-019-57147-7. Sci Rep. 2020. PMID: 31937874 Free PMC article.
-
R213G polymorphism in SOD3 protects against bleomycin-induced inflammation and attenuates induction of proinflammatory pathways.Physiol Genomics. 2018 Sep 1;50(9):807-816. doi: 10.1152/physiolgenomics.00053.2018. Epub 2018 Jul 13. Physiol Genomics. 2018. PMID: 30004839 Free PMC article.
References
-
- Archer SL, Marsboom G, Kim GH, Zhang HJ, Toth PT, Svensson EC, Dyck JR, Gomberg-Maitland M, Thebaud B, Husain AN, Cipriani N, Rehman J. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: a basis for excessive cell proliferation and a new therapeutic target. Circulation 121: 2661–2671, 2010. - PMC - PubMed
-
- Bogaard HJ, Mizuno S, Hussaini AA, Toldo S, Abbate A, Kraskauskas D, Kasper M, Natarajan R, Voelkel NF. Suppression of histone deacetylases worsens right ventricular dysfunction after pulmonary artery banding in rats. Am J Respir Crit Care Med 183: 1402–1410, 2011. - PubMed
-
- Cavasin MA, Demos-Davies K, Horn TR, Walker LA, Lemon DD, Birdsey N, Weiser-Evans MC, Harral J, Irwin DC, Anwar A, Yeager ME, Li M, Watson PA, Nemenoff RA, Buttrick PM, Stenmark KR, McKinsey TA. Selective class I histone deacetylase inhibition suppresses hypoxia-induced cardiopulmonary remodeling through an antiproliferative mechanism. Circ Res 110: 739–748, 2012. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

