CNS tau efflux via exosomes is likely increased in Parkinson's disease but not in Alzheimer's disease
- PMID: 27234211
- PMCID: PMC5107127
- DOI: 10.1016/j.jalz.2016.04.003
CNS tau efflux via exosomes is likely increased in Parkinson's disease but not in Alzheimer's disease
Abstract
Introduction: Alzheimer's disease (AD) and Parkinson's disease (PD) involve tau pathology. Tau is detectable in blood, but its clearance from neuronal cells and the brain is poorly understood.
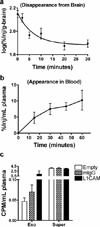
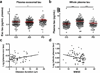
Methods: Tau efflux from the brain to the blood was evaluated by administering radioactively labeled and unlabeled tau intracerebroventricularly in wild-type and tau knock-out mice, respectively. Central nervous system (CNS)-derived tau in L1CAM-containing exosomes was further characterized extensively in human plasma, including by single molecule array technology with 303 subjects.
Results: The efflux of Tau, including a fraction via CNS-derived L1CAM exosomes, was observed in mice. In human plasma, tau was explicitly identified within L1CAM exosomes. In contrast to AD patients, L1CAM exosomal tau was significantly higher in PD patients than controls and correlated with cerebrospinal fluid tau.
Conclusions: Tau is readily transported from the brain to the blood. The mechanisms of CNS tau efflux are likely different between AD and PD.
Keywords: Alzheimer's disease; Biomarkers; Blood plasma; Central nervous system protein efflux; Central nervous system-derived exosomes; Parkinson's disease; Tau.
Copyright © 2016 The Alzheimer's Association. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Nothing to report.
Figures
References
-
- Spillantini MG, Goedert M. Tau pathology and neurodegeneration. Lancet Neurol. 2013;12:609–622. - PubMed
-
- Simon D, Garcia-Garcia E, Gomez-Ramos A, Falcon-Perez JM, Diaz-Hernandez M, Hernandez F, et al. Tau overexpression results in its secretion via membrane vesicles. Neurodegener Dis. 2012;10:73–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS057567/NS/NINDS NIH HHS/United States
- P50 AG005131/AG/NIA NIH HHS/United States
- I01 BX000531/BX/BLRD VA/United States
- R01 NS065070/NS/NINDS NIH HHS/United States
- U01 NS091272/NS/NINDS NIH HHS/United States
- P30 AG008017/AG/NIA NIH HHS/United States
- P30 ES007033/ES/NIEHS NIH HHS/United States
- P50 NS062684/NS/NINDS NIH HHS/United States
- R01 AG033398/AG/NIA NIH HHS/United States
- R21 NS085425/NS/NINDS NIH HHS/United States
- U01 NS082137/NS/NINDS NIH HHS/United States
- R01 ES016873/ES/NIEHS NIH HHS/United States
- R01 ES019277/ES/NIEHS NIH HHS/United States
- P50 AG005136/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

