Ubiquitin-like domains can target to the proteasome but proteolysis requires a disordered region
- PMID: 27234297
- PMCID: PMC4884787
- DOI: 10.15252/embj.201593147
Ubiquitin-like domains can target to the proteasome but proteolysis requires a disordered region
Abstract
Ubiquitin and some of its homologues target proteins to the proteasome for degradation. Other ubiquitin-like domains are involved in cellular processes unrelated to the proteasome, and proteins containing these domains remain stable in the cell. We find that the 10 yeast ubiquitin-like domains tested bind to the proteasome, and that all 11 identified domains can target proteins for degradation. Their apparent proteasome affinities are not directly related to their stabilities or functions. That is, ubiquitin-like domains in proteins not part of the ubiquitin proteasome system may bind the proteasome more tightly than domains in proteins that are bona fide components. We propose that proteins with ubiquitin-like domains have properties other than proteasome binding that confer stability. We show that one of these properties is the absence of accessible disordered regions that allow the proteasome to initiate degradation. In support of this model, we find that Mdy2 is degraded in yeast when a disordered region in the protein becomes exposed and that the attachment of a disordered region to Ubp6 leads to its degradation.
Keywords: degradation; proteasome; ubiquitin‐like domain.
© 2016 The Authors.
Figures
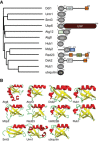
Phylogenetic relationship of UbL domains in S. cerevisiae and schematic representations of the corresponding UbL proteins. Sequences were clustered and mapped onto a N‐J tree by ClustalX2 (Larkin et al, 2007) and the PHYLIP package (Felsenstein, 1989). ASP, aspartyl protease domain; UBA, ubiquitin‐associated domain; USP, ubiquitin‐specific protease domain; DD, dimerization domain; RBD, Rad4‐binding domain; ST2, Sti1/Sti1 domain pair.
Structural models of UbL domains generated by PyMOL (The PyMOL Molecular Graphics System, Version 0.99 Schrödinger, LLC). PDB IDs: Atg8 2ZPN, Atg12 3W1S, Dsk2 2BWF, Hub1 1M94, Mdy2 4GOC, Rad23 3M62, hHR23B 1P1A, Rub1 1BT0, Smt3 1EUV, Urm1 2PK0, ubiquitin 1UBQ.
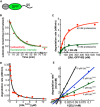
Sketch of UbLRad23‐GFP‐95 consisting of the UbL domain of S. cerevisiae Rad23, followed by superfolder GFP and a 95‐amino acid‐long tail derived from S. cerevisiae cytochrome b 2.
In vitro degradation of UbLRad23‐GFP‐95 by purified S. cerevisiae proteasome. The graph plots the amount of substrate over time, estimated by fluorescence intensity monitored by plate reader (green) or electronic autoradiography of SDS–PAGE gel bands (red), normalized to the initial substrate amount as described in Materials and Methods.
Michaelis–Menten analysis of UbLRad23‐GFP‐95 degradation by different concentrations of purified S. cerevisiae proteasome (green, 10 nM; red, 40 nM).
UbLRad23 inhibits UbLRad23‐GFP‐95 degradation. The initial degradation rates of UbLRad23‐GFP‐95 at different concentrations of purified UbLRad23 domain are plotted and fitted to the equation describing competitive inhibition.
The UbLDsk2 domain is a competitive inhibitor of UbLRad23‐GFP‐95 degradation. Lineweaver–Burk plot of UbLRad23‐GFP‐95 degradation with different concentrations of purified UbLDsk2 domain.
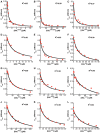
- A–L
Initial degradation rates of UbLRad23‐GFP‐95 in the presence of different concentrations of purified UbL domains plotted against the competitor concentration and fitted to the equation describing competitive inhibition. The UbL domains were from yeast Rad23 (A), Dsk2 (C), Atg8 (D), Hub1 (E), ubiquitin (F), Ddi1 (G), Ubp6 (H), Urm1 (I), Mdy2 (J), Rub1 (K), Smt3 (L), and hHR23B (B). Graphs show one representative dataset of at least three independent experiments.
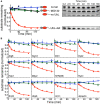
- A, B
In vitro degradation of model proteins by purified S. cerevisiae proteasome. The model proteins (UbL‐DHFR‐95) consisted of an N‐terminal UbL domain, followed by an E. coli DHFR domain and a 95‐amino acid tail derived from S. cerevisiae cytochrome b 2. UbL‐DHFR‐95 proteins were degraded by proteasome (red solid circle) and stabilized by removing the UbL domain (no UbL, black triangles), by removing the 95‐amino acid tail (no tail, blue diamonds), or by proteasome inhibitor (MG132, green squares). (A) Degradation of model proteins containing the UbL domain of S. cerevisiae Rad23. (B) Degradation of model proteins containing other UbL domains or ubiquitin. The graphs plot the amount of substrate estimated by electronic autoradiography in SDS–PAGE gel bands over time as normalized to the initial substrate amount as described in Materials and Methods. Data points represent mean values determined from at least three repeat experiments; error bars indicate s.e.m.

Schematic representation of yeast growth assay. The model proteins consisted of an UbL domain and S. cerevisiae imidazoleglycerol‐phosphate dehydratase (His3), followed by stop codon (no tail) or a 51‐amino acid tail derived from subunit 9 of the F o component of the Neurospora crassa ATPase (Su9) at the C‐terminus. In his3 mutant cells, the absence of His3 protein caused a growth defect when grown on selective media. Stable His3 proteins escaped proteasome degradation and rescued the his3 mutant cells from the growth defect.
UbL domains mediated the degradation of His3 fusion proteins with a Su9 tail and affected yeast growth under selective condition (+3‐AT, −his). Replacing UbL domains with DHFR domains rescued the growth defect. Cells expressing His3 fusion proteins in late log phase were serially diluted and stamped on selective plates. The plates were incubated at 30°C for 3 days for imaging.
In the E1 temperature‐sensitive strain uba1‐204, the cellular abundance of UbL‐His3‐tail proteins was similar at the permissive (30°C) and restrictive (37°C) temperatures. In contrast, a His3 fusion protein containing a classic N‐end rule degron was stabilized at the restrictive temperature (37°C), showing that its degradation depended on the ubiquitination machinery. Bortezomib stabilized both the UbL‐His3‐tail and N‐degron‐His3‐tail proteins.
UbL‐His3 proteins without initiation region were stable and not affected by the shift to the restrictive temperature or treatment with bortezomib.

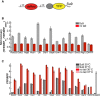
Fluorescence‐based degradation assay in S. cerevisiae. The substrate proteins consisted of a UbL domain, followed by a yellow fluorescent protein (YFP) domain and a disordered tail (51 amino acids derived from subunit 9 of the F o component of the Neurospora crassa ATPase, Su9). Control substrates lacked the disordered tail. Substrates and a red fluorescent protein were expressed from consecutive constitutive promoters (pACT1) on the same CEN plasmid (YCplac33). Fluorescence profiles of cells expressing different substrates monitored by flow cytometry. UbLRad23 targeted YFP‐Su9 protein for degradation. Removing the Su9 tail or replacing UbLRad23 with a DHFR domain stabilized YFP protein in cells.
UbL domains targeted YFP substrates to degradation in the presence of an initiation region. The Y‐axis plots the abundance of the UbL‐YFP‐tail proteins normalized by a translation control, which is the same UbL‐YFP protein without the tail. The ratio is equal to one for domains that are not recognized by the proteasome, and smaller than one if the UbL is recognized by the proteasome and the UbL‐YFP‐tail protein is degraded. YFP fluorescence was also corrected for plasmid copy number using RFP fluorescence. The median of YFP/RFP ratio for each construct was calculated from 10,000 cells collected in one flow cytometry run and reflected the abundance of YFP substrates in yeast cells as described in Materials and Methods. Data points represent the mean values determined from at least three repeat experiments; error bars indicate s.e.m.
Schematic representation of UbL‐YFP constructs. The fluorescent substrates consisted of a UbL domain, followed by a yellow fluorescent protein (YFP) domain and a tail (51 amino acids derived from subunit 9 of the F o component of the Neurospora crassa ATPase, Su9) or a stop codon at the C‐terminus. dsRed Express2 (expression control) and UbL‐YFP‐Su9 (substrate) were expressed from the consecutive constitutive ACT1 promoters on the same CEN plasmid (YCplac33).
Stabilization of UbL‐YFP substrates with tails (Su9, gray) or without tails (no tail, red) by proteasome inhibitor (bortezomib) treatment. The extent of recovery was calculated as the fraction of the YFP/RFP ratios of cells expressing each construct after bortezomib treatment divided by the YFP/RFP ratios for cells with the same construct after DMSO treatment.
Levels of UbL‐YFP proteins with tails (Su9, gray) or without tails (no tail, red) in the E1 temperature‐sensitive strain uba1‐204 at the permissive temperature (30°C; UbL‐YFP‐Su9, dark gray; UbL‐YFP, dark red) and the restrictive temperature (37°C; UbL‐YFP‐Su9, light gray; UbL‐YFP, light red). The median of YFP/RFP value for each construct was calculated from 10,000 cells collected in one flow cytometry run and was used to indicate the concentration of YFP substrate in yeast cells as described in Materials and Methods.
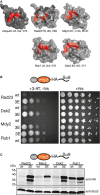
The proteasome‐interacting residues on ubiquitin and the UbL domains of yeast Rad23, Mdy2, Rub1, and Dsk2 that were mutated to glutamic acid residues (3E mutations) in this study are shown in red. Structural models of ubiquitin and UbL domains generated by PyMol (version 0.99). PDB IDs: Dsk2 2BWF; Mdy2 4GOC; Rad23 3M62; Rub1 1BT0; ubiquitin 1UBQ.
3E mutations rescued the growth defect of his3 strains expressing UbL‐His3‐tail constructs under selective conditions (+3‐AT, −his). Cells expressing the His3 fusion proteins in late log phase were serially diluted and stamped on selective plates. The plates were incubated at 30°C for 3 days for imaging.
3E mutations stabilized UbL‐His3‐tail proteins in yeast cells. Protein levels were determined by Western blotting for the HA‐tag and for Scs2 protein as a loading control in SDS–PAGE gels of S. cerevisiae protein extracts.

Schematic representation of S. cerevisiae Mdy2. Structural models of the N‐terminal domain (NTD) of Mdy2 (amino acids 1–59, green), with its binding partner Get4 (red, PDB ID: 2WPV), the central UbL domain of Mdy2 (amino acids 74–148, gray, PDB ID: 4GOC), and the C‐terminal dimerization domain (DD) of Mdy2 (amino acids 152–212, blue, PDB ID: 2LNZ).
In vitro degradation of Mdy2 proteins by purified S. cerevisiae proteasome. Mdy2 (red solid circles) was degraded by the proteasome; removing the NTD (Mdy2ΔN, black solid circles) stabilized the protein but attaching a C‐terminal tail to the truncated protein (Mdy2ΔN‐95, blue solid circles) restored degradation. Mdy2 (red solid circles) was also stabilized by the addition of its binding partner Get4 (red open squares, dashed line) or UbLMdy2 as a competitor for proteasome binding (red open diamonds, dotted line). When Get4 binding buried the NTD, fusion of a tail to the C‐terminus of Mdy2 (green open squares, dotted line) restored degradation. The graph plots the amount of substrate remaining estimated by electronic autoradiography in SDS–PAGE gel bands over time as normalized to the initial amount of substrate as described in Materials and Methods. Data points represent the mean values determined from at least three repeat experiments; error bars indicate s.e.m.
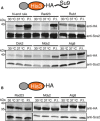
Degradation of Mdy2 in vivo. Steady‐state levels of overexpressed C‐terminally HA‐tagged full‐length Mdy2 and a mutant in which the NTD was deleted (Mdy2ΔN) in S. cerevisiae, in the absence of Get4 or in the presence of endogenous Get4 or overexpressed N‐terminally Flag‐tagged Get4. Protein levels were determined by Western blotting for the HA‐ or Flag‐tag, or for the protein Scs2 as a loading control, of SDS‐PAGE gels of S. cerevisiae protein extracts. The proteasome was inhibited by bortezomib as indicated.
Steady‐state levels of endogenous Mdy2 in Get4 wild‐type (GET4) or deletion (get4Δ) S. cerevisiae strains in the presence or absence of the proteasome inhibitor bortezomib. Protein levels were determined by Western blotting for Mdy2, or Scs2 as the loading control, of SDS–PAGE gels of S. cerevisiae protein extracts.
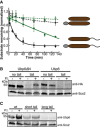
In vitro degradation of Ubp6 proteins by purified S. cerevisiae proteasome. Full‐length Ubp6 (Ubp6, black open circles) and a UbL deletion mutant (Ubp6ΔN, green open circles) escaped degradation. Attachment of a C‐terminal 95‐amino acid tail derived from S. cerevisiae cytochrome b 2 allowed the proteasome to degrade the Ubp6 proteins (Ubp6‐95, black solid circles and Ubp6ΔN‐95, green solid circles). The graph plots the amount of substrate estimated by electronic autoradiography in SDS–PAGE gel bands over time as normalized to the initial substrate amount as described in Materials and Methods. Data points represent the mean values determined from at least three repeat experiments; error bars indicate s.e.m.
Degradation of Ubp6 proteins in vivo. Steady‐state levels of N‐terminally HA‐tagged Ubp6 mutants in S. cerevisiae. N‐terminally HA‐tagged Ubp6 and Ubp6∆N with or without a Su9‐tail attached to their C‐termini were overexpressed in yeast. Protein levels were determined by Western blotting for the HA‐tag, or the protein Scs2 as a loading control, of SDS–PAGE gels of S. cerevisiae protein extracts. The proteasome was inhibited by bortezomib where indicated.
Steady‐state levels of endogenous Ubp6 and Ubp6 derivatives with short (19 amino acid) or long (54 amino acid) tails at their C‐termini. Protein levels were determined by Western blotting for Ubp6, or Scs2 as a loading control, of SDS–PAGE gels of S. cerevisiae protein extracts. The proteasome was inhibited by bortezomib as indicated.
Similar articles
-
Proteins containing ubiquitin-like (Ubl) domains not only bind to 26S proteasomes but also induce their activation.Proc Natl Acad Sci U S A. 2020 Mar 3;117(9):4664-4674. doi: 10.1073/pnas.1915534117. Epub 2020 Feb 18. Proc Natl Acad Sci U S A. 2020. PMID: 32071216 Free PMC article.
-
Structural characterization of the interaction of Ubp6 with the 26S proteasome.Proc Natl Acad Sci U S A. 2015 Jul 14;112(28):8626-31. doi: 10.1073/pnas.1510449112. Epub 2015 Jun 30. Proc Natl Acad Sci U S A. 2015. PMID: 26130806 Free PMC article.
-
Ubp6 deubiquitinase controls conformational dynamics and substrate degradation of the 26S proteasome.Nat Struct Mol Biol. 2015 Sep;22(9):712-9. doi: 10.1038/nsmb.3075. Epub 2015 Aug 24. Nat Struct Mol Biol. 2015. PMID: 26301997 Free PMC article.
-
Deubiquitinating enzymes are IN/(trinsic to proteasome function).Curr Protein Pept Sci. 2004 Jun;5(3):201-11. doi: 10.2174/1389203043379756. Curr Protein Pept Sci. 2004. PMID: 15188770 Review.
-
Mitochondrial quality control by the ubiquitin-proteasome system.Biochem Soc Trans. 2011 Oct;39(5):1509-13. doi: 10.1042/BST0391509. Biochem Soc Trans. 2011. PMID: 21936843 Review.
Cited by
-
Degron masking outlines degronons, co-degrading functional modules in the proteome.Commun Biol. 2022 May 11;5(1):445. doi: 10.1038/s42003-022-03391-z. Commun Biol. 2022. PMID: 35545699 Free PMC article.
-
Structure and Function of the 26S Proteasome.Annu Rev Biochem. 2018 Jun 20;87:697-724. doi: 10.1146/annurev-biochem-062917-011931. Epub 2018 Apr 13. Annu Rev Biochem. 2018. PMID: 29652515 Free PMC article. Review.
-
Proteasomal conformation controls unfolding ability.Proc Natl Acad Sci U S A. 2021 Jun 22;118(25):e2101004118. doi: 10.1073/pnas.2101004118. Proc Natl Acad Sci U S A. 2021. PMID: 34161281 Free PMC article.
-
How to target membrane proteins for degradation: Bringing GPCRs into the TPD fold.J Biol Chem. 2024 Dec;300(12):107926. doi: 10.1016/j.jbc.2024.107926. Epub 2024 Oct 23. J Biol Chem. 2024. PMID: 39454955 Free PMC article. Review.
-
Mode of targeting to the proteasome determines GFP fate.J Biol Chem. 2020 Nov 20;295(47):15892-15901. doi: 10.1074/jbc.RA120.015235. Epub 2020 Sep 10. J Biol Chem. 2020. PMID: 32913119 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

