Suppression of glymphatic fluid transport in a mouse model of Alzheimer's disease
- PMID: 27234656
- PMCID: PMC4980916
- DOI: 10.1016/j.nbd.2016.05.015
Suppression of glymphatic fluid transport in a mouse model of Alzheimer's disease
Abstract
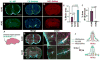
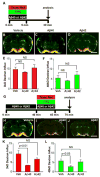
Glymphatic transport, defined as cerebrospinal fluid (CSF) peri-arterial inflow into brain, and interstitial fluid (ISF) clearance, is reduced in the aging brain. However, it is unclear whether glymphatic transport affects the distribution of soluble Aβ in Alzheimer's disease (AD). In wild type mice, we show that Aβ40 (fluorescently labeled Aβ40 or unlabeled Aβ40), was distributed from CSF to brain, via the peri-arterial space, and associated with neurons. In contrast, Aβ42 was mostly restricted to the peri-arterial space due mainly to its greater propensity to oligomerize when compared to Aβ40. Interestingly, pretreatment with Aβ40 in the CSF, but not Aβ42, reduced CSF transport into brain. In APP/PS1 mice, a model of AD, with and without extensive amyloid-β deposits, glymphatic transport was reduced, due to the accumulation of toxic Aβ species, such as soluble oligomers. CSF-derived Aβ40 co-localizes with existing endogenous vascular and parenchymal amyloid-β plaques, and thus, may contribute to the progression of both cerebral amyloid angiopathy and parenchymal Aβ accumulation. Importantly, glymphatic failure preceded significant amyloid-β deposits, and thus, may be an early biomarker of AD. By extension, restoring glymphatic inflow and ISF clearance are potential therapeutic targets to slow the onset and progression of AD.
Keywords: AQP4; Alzheimer's disease; Amyloid-β; Astrocytes; Brain ISF clearance; CSF; Clearance; Convective ISF flow; Glymphatic pathways; Lymphatic system.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



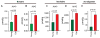

References
-
- Abbott NJ, et al. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

