Genetic causes of hypomagnesemia, a clinical overview
- PMID: 27234911
- PMCID: PMC5440500
- DOI: 10.1007/s00467-016-3416-3
Genetic causes of hypomagnesemia, a clinical overview
Abstract
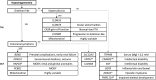
Magnesium is essential to the proper functioning of numerous cellular processes. Magnesium ion (Mg2+) deficits, as reflected in hypomagnesemia, can cause neuromuscular irritability, seizures and cardiac arrhythmias. With normal Mg2+ intake, homeostasis is maintained primarily through the regulated reabsorption of Mg2+ by the thick ascending limb of Henle's loop and distal convoluted tubule of the kidney. Inadequate reabsorption results in renal Mg2+ wasting, as evidenced by an inappropriately high fractional Mg2+ excretion. Familial renal Mg2+ wasting is suggestive of a genetic cause, and subsequent studies in these hypomagnesemic families have revealed over a dozen genes directly or indirectly involved in Mg2+ transport. Those can be classified into four groups: hypercalciuric hypomagnesemias (encompassing mutations in CLDN16, CLDN19, CASR, CLCNKB), Gitelman-like hypomagnesemias (CLCNKB, SLC12A3, BSND, KCNJ10, FYXD2, HNF1B, PCBD1), mitochondrial hypomagnesemias (SARS2, MT-TI, Kearns-Sayre syndrome) and other hypomagnesemias (TRPM6, CNMM2, EGF, EGFR, KCNA1, FAM111A). Although identification of these genes has not yet changed treatment, which remains Mg2+ supplementation, it has contributed enormously to our understanding of Mg2+ transport and renal function. In this review, we discuss general mechanisms and symptoms of genetic causes of hypomagnesemia as well as the specific molecular mechanisms and clinical phenotypes associated with each syndrome.
Keywords: Distal convoluted tubule; Hereditary; Homeostasis; Kidney; Magnesium; Thick ascending limb of Henle’s loop.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

References
-
- Brunette MG, Vigneault N, Carriere S. Micropuncture study of magnesium transport along the nephron in the young rat. Am J Physiol. 1974;227:891–896. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

