Novel mutations in RASGRP2, which encodes CalDAG-GEFI, abrogate Rap1 activation, causing platelet dysfunction
- PMID: 27235135
- PMCID: PMC5009515
- DOI: 10.1182/blood-2015-11-683102
Novel mutations in RASGRP2, which encodes CalDAG-GEFI, abrogate Rap1 activation, causing platelet dysfunction
Abstract
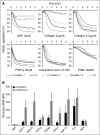
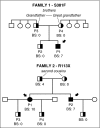
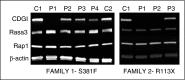
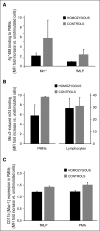
In addition to mutations in ITG2B or ITGB3 genes that cause defective αIIbβ3 expression and/or function in Glanzmann's thrombasthenia patients, platelet dysfunction can be a result of genetic variability in proteins that mediate inside-out activation of αIIbβ3 The RASGRP2 gene is strongly expressed in platelets and neutrophils, where its encoded protein CalDAG-GEFI facilitates the activation of Rap1 and subsequent activation of integrins. We used next-generation sequencing (NGS) and whole-exome sequencing (WES) to identify 2 novel function-disrupting mutations in RASGRP2 that account for bleeding diathesis and platelet dysfunction in 2 unrelated families. By using a panel of 71 genes, we identified a homozygous change (c.1142C>T) in exon 10 of RASGRP2 in a 9-year-old child of Chinese origin (family 1). This variant led to a p.Ser381Phe substitution in the CDC25 catalytic domain of CalDAG-GEFI. In 2 Spanish siblings from family 2, WES identified a nonsense homozygous variation (c.337C>T) (p.Arg113X) in exon 5 of RASGRP2 CalDAG-GEFI expression was markedly reduced in platelets from all patients, and by using a novel in vitro assay, we found that the nucleotide exchange activity was dramatically reduced in CalDAG-GEFI p.Ser381Phe. Platelets from homozygous patients exhibited agonist-specific defects in αIIbβ3 integrin activation and aggregation. In contrast, α- and δ-granule secretion, platelet spreading, and clot retraction were not markedly affected. Integrin activation in the patients' neutrophils was also impaired. These patients are the first cases of a CalDAG-GEFI deficiency due to homozygous RASGRP2 mutations that are linked to defects in both leukocyte and platelet integrin activation.
© 2016 by The American Society of Hematology.
Figures


Comment in
-
Inherited CalDAG-GEFI deficiency.Blood. 2016 Sep 1;128(9):1165-7. doi: 10.1182/blood-2016-07-719906. Blood. 2016. PMID: 27587867 No abstract available.
References
-
- Bolton-Maggs PH, Chalmers EA, Collins PW, et al. UKHCDO. A review of inherited platelet disorders with guidelines for their management on behalf of the UKHCDO. Br J Haematol. 2006;135(5):603–633. - PubMed
-
- Nurden AT, Nurden P. Congenital platelet disorders and understanding of platelet function. Br J Haematol. 2014;165(2):165–178. - PubMed
-
- Nurden AT, Pillois X, Fiore M, et al. Expanding the Mutation Spectrum Affecting αIIbβ3 Integrin in Glanzmann Thrombasthenia: Screening of the ITGA2B and ITGB3 Genes in a Large International Cohort. Hum Mutat. 2015;36(5):548–561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

