Distal Hydrogen-bonding Interactions in Ligand Sensing and Signaling by Mycobacterium tuberculosis DosS
- PMID: 27235395
- PMCID: PMC4965560
- DOI: 10.1074/jbc.M116.724815
Distal Hydrogen-bonding Interactions in Ligand Sensing and Signaling by Mycobacterium tuberculosis DosS
Abstract
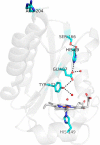
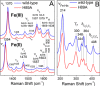
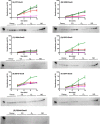
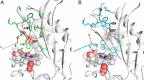
Mycobacterium tuberculosis DosS is critical for the induction of M. tuberculosis dormancy genes in response to nitric oxide (NO), carbon monoxide (CO), or hypoxia. These environmental stimuli, which are sensed by the DosS heme group, result in autophosphorylation of a DosS His residue, followed by phosphotransfer to an Asp residue of the response regulator DosR. To clarify the mechanism of gaseous ligand recognition and signaling, we investigated the hydrogen-bonding interactions of the iron-bound CO and NO ligands by site-directed mutagenesis of Glu-87 and His-89. Autophosphorylation assays and molecular dynamics simulations suggest that Glu-87 has an important role in ligand recognition, whereas His-89 is essential for signal transduction to the kinase domain, a process for which Arg-204 is important. Mutation of Glu-87 to Ala or Gly rendered the protein constitutively active as a kinase, but with lower autophosphorylation activity than the wild-type in the Fe(II) and the Fe(II)-CO states, whereas the E87D mutant had little kinase activity except for the Fe(II)-NO complex. The H89R mutant exhibited attenuated autophosphorylation activity, although the H89A and R204A mutants were inactive as kinases, emphasizing the importance of these residues in communication to the kinase core. Resonance Raman spectroscopy of the wild-type and H89A mutant indicates the mutation does not alter the heme coordination number, spin state, or porphyrin deformation state, but it suggests that interdomain interactions are disrupted by the mutation. Overall, these results confirm the importance of the distal hydrogen-bonding network in ligand recognition and communication to the kinase domain and reveal the sensitivity of the system to subtle differences in the binding of gaseous ligands.
Keywords: Mycobacterium tuberculosis; biosensor; carbon monoxide; heme; hydrogen bonding network; molecular dynamics; nitric oxide; oxygen binding; resonance Raman.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Dye C., Scheele S., Dolin P., Pathania V., and Raviglione M. C. (1999) Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. J. Am. Med. Assoc. 282, 677–686 - PubMed
-
- Parrish N. M., Dick J. D., and Bishai W. R. (1998) Mechanisms of latency in Mycobacterium tuberculosis. Trends Microbiol. 6, 107–112 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

