Regulation of Connexin43 Function and Expression by Tyrosine Kinase 2
- PMID: 27235399
- PMCID: PMC4957067
- DOI: 10.1074/jbc.M116.727008
Regulation of Connexin43 Function and Expression by Tyrosine Kinase 2
Abstract
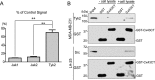
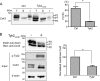
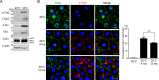
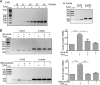
Connexin43 (Cx43) assembly and degradation, the regulation of electrical and metabolic coupling, as well as modulating the interaction with other proteins, involve phosphorylation. Here, we identified and characterized the biological significance of a novel tyrosine kinase that phosphorylates Cx43, tyrosine kinase 2 (Tyk2). Activation of Tyk2 led to a decrease in Cx43 gap junction communication by increasing the turnover rate of Cx43 from the plasma membrane. Tyk2 directly phosphorylated Cx43 residues Tyr-247 and Tyr-265, leading to indirect phosphorylation on residues Ser-279/Ser-282 (MAPK) and Ser-368 (PKC). Although this phosphorylation pattern is similar to what has been observed following Src activation, the response caused by Tyk2 occurred when Src was inactive in NRK cells. Knockdown of Tyk2 at the permissive temperature (active v-Src) in LA-25 cells decreased Cx43 phosphorylation, indicating that although activation of Tyk2 and v-Src leads to phosphorylation of the same Cx43CT residues, they are not identical in level at each site. Additionally, angiotensin II activation of Tyk2 increased the intracellular protein level of Cx43 via STAT3. These findings indicate that, like Src, Tyk2 can also inhibit gap junction communication by phosphorylating Cx43.
Keywords: Western blot; cell biology; cell signaling; gap junction; phosphorylation.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures








References
-
- Solan J. L., and Lampe P. D. (2005) Connexin phosphorylation as a regulatory event linked to gap junction channel assembly. Biochim. Biophys. Acta 1711, 154–163 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

