Mitochondria-associated ER membranes and Alzheimer disease
- PMID: 27235807
- PMCID: PMC5390896
- DOI: 10.1016/j.gde.2016.04.006
Mitochondria-associated ER membranes and Alzheimer disease
Abstract
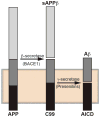
The series of events underlying the pathogenesis of Alzheimer disease (AD) in unknown. The most widely accepted hypothesis is called the amyloid cascade, based on the observation that the brains of AD patients contain high levels of extracellular plaques, composed mainly of β-amyloid (Aβ), and intracellular tangles, composed of hyperphosphorylated forms of the microtubule-associated protein tau. However, AD is also characterized by other features, including aberrant cholesterol, phospholipid, and calcium metabolism, and mitochondrial dysfunction, all ostensibly unrelated to plaque and tangle formation. Notably, these 'other' aspects of AD pathology are functions related to mitochondria-associated ER membranes (MAM), a subdomain of the endoplasmic reticulum (ER) that is apposed to, and communicates with, mitochondria. Given the potential relationship between MAM and AD, we explored the possibility that perturbed MAM function might play a role in AD pathogenesis. We found that γ-secretase activity, which processes the amyloid precursor protein to generate Aβ, is located predominantly in the MAM, and that ER-mitochondrial apposition and MAM function are increased significantly in cells from AD patients. These observations may help explain not only the aberrant Aβ production, but also many of the 'other' biochemical and morphological features of the disease. Based on these, and other, data we propose that AD is fundamentally a disorder of ER-mitochondrial hyperconnectivity.
Copyright © 2016 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Querfurth HW, LaFerla FM. Alzheimer’s disease. New Eng J Med. 2010;362:329–344. - PubMed
-
- Armstrong RA, Lantos PL, Cairns NJ. What determines the molecular composition of abnormal protein aggregates in neurodegenerative disease? Neuropathology. 2008;28:351–365. - PubMed
-
- Atwood CS, Martins RN, Smith MA, Perry G. Senile plaque composition and posttranslational modification of amyloid-β peptide and associated proteins. Peptides. 2002;23:1343–1350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

