The importance of nerve microenvironment for schwannoma development
- PMID: 27236462
- PMCID: PMC4947119
- DOI: 10.1007/s00401-016-1583-8
The importance of nerve microenvironment for schwannoma development
Abstract
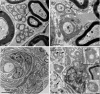
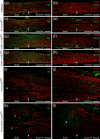
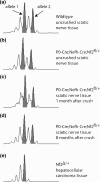
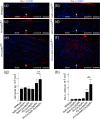
Schwannomas are predominantly benign nerve sheath neoplasms caused by Nf2 gene inactivation. Presently, treatment options are mainly limited to surgical tumor resection due to the lack of effective pharmacological drugs. Although the mechanistic understanding of Nf2 gene function has advanced, it has so far been primarily restricted to Schwann cell-intrinsic events. Extracellular cues determining Schwann cell behavior with regard to schwannoma development remain unknown. Here we show pro-tumourigenic microenvironmental effects on Schwann cells where an altered axonal microenvironment in cooperation with injury signals contribute to a persistent regenerative Schwann cell response promoting schwannoma development. Specifically in genetically engineered mice following crush injuries on sciatic nerves, we found macroscopic nerve swellings in mice with homozygous nf2 gene deletion in Schwann cells and in animals with heterozygous nf2 knockout in both Schwann cells and axons. However, patient-mimicking schwannomas could only be provoked in animals with combined heterozygous nf2 knockout in Schwann cells and axons. We identified a severe re-myelination defect and sustained macrophage presence in the tumor tissue as major abnormalities. Strikingly, treatment of tumor-developing mice after nerve crush injury with medium-dose aspirin significantly decreased schwannoma progression in this disease model. Our results suggest a multifactorial concept for schwannoma formation-emphasizing axonal factors and mechanical nerve irritation as predilection site for schwannoma development. Furthermore, we provide evidence supporting the potential efficacy of anti-inflammatory drugs in the treatment of schwannomas.
Keywords: Crush injury, tissue inflammation; Microenvironment; NF2; Neurofibromatosis type 2; Schwannoma; Sciatic nerve; Tumor induction.
Figures




References
-
- Boin A, Couvelard A, Couderc C, Brito I, Filipescu D, Kalamarides M, Bedossa P, De Koning L, Danelsky C, Dubois T, et al. Proteomic screening identifies a YAP-driven signaling network linked to tumor cell proliferation in human schwannomas. Neuro-oncology. 2014;16:1196–1209. doi: 10.1093/neuonc/nou020. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

