Von Willebrand factor regulates complement on endothelial cells
- PMID: 27236750
- PMCID: PMC6591736
- DOI: 10.1016/j.kint.2016.03.023
Von Willebrand factor regulates complement on endothelial cells
Abstract
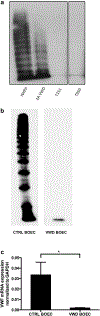
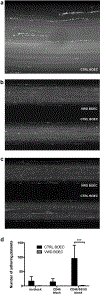
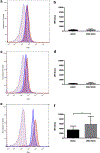
Atypical hemolytic uremic syndrome and thrombotic thrombocytopenic purpura have traditionally been considered separate entities. Defects in the regulation of the complement alternative pathway occur in atypical hemolytic uremic syndrome, and defects in the cleavage of von Willebrand factor (VWF)-multimers arise in thrombotic thrombocytopenic purpura. However, recent studies suggest that both entities are related as defects in the disease-causing pathways overlap or show functional interactions. Here we investigate the possible functional link of VWF-multimers and the complement system on endothelial cells. Blood outgrowth endothelial cells (BOECs) were obtained from 3 healthy individuals and 2 patients with Type 3 von Willebrand disease lacking VWF. Cells were exposed to a standardized complement challenge via the combination of classical and alternative pathway activation and 50% normal human serum resulting in complement fixation to the endothelial surface. Under these conditions we found the expected release of VWF-multimers causing platelet adhesion onto BOECs from healthy individuals. Importantly, in BOECs derived from patients with von Willebrand disease complement C3c deposition and cytotoxicity were more pronounced than on BOECs derived from normal individuals. This is of particular importance as primary glomerular endothelial cells display a heterogeneous expression pattern of VWF with overall reduced VWF abundance. Thus, our results support a mechanistic link between VWF-multimers and the complement system. However, our findings also identify VWF as a new complement regulator on vascular endothelial cells and suggest that VWF has a protective effect on endothelial cells and complement-mediated injury.
Keywords: atypical hemolytic uremic syndrome; blood outgrowth endothelial cells; complement; thrombotic microangiopathy; thrombotic thrombocytopenic purpura; von Willebrand factor.
Copyright © 2016 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DISCLOSURE
All the authors declared no competing interests.
Figures




References
-
- Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:1676–1687. - PubMed
-
- Tsai HM. Untying the knot of thrombotic thrombocytopenic purpura and atypical hemolytic uremic syndrome. Am J Med. 2013;126:200–209. - PubMed
-
- Chapin J, Eyler S, Smith R, et al. Complement factor H mutations are present in ADAMTS13-deficient, ticlopidine-associated thrombotic microangiopathies. Blood. 2013;121:4012–4013. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

