Trypanosoma brucei Parasites Occupy and Functionally Adapt to the Adipose Tissue in Mice
- PMID: 27237364
- PMCID: PMC4906371
- DOI: 10.1016/j.chom.2016.05.002
Trypanosoma brucei Parasites Occupy and Functionally Adapt to the Adipose Tissue in Mice
Abstract
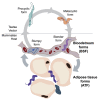
Trypanosoma brucei is an extracellular parasite that causes sleeping sickness. In mammalian hosts, trypanosomes are thought to exist in two major niches: early in infection, they populate the blood; later, they breach the blood-brain barrier. Working with a well-established mouse model, we discovered that adipose tissue constitutes a third major reservoir for T. brucei. Parasites from adipose tissue, here termed adipose tissue forms (ATFs), can replicate and were capable of infecting a naive animal. ATFs were transcriptionally distinct from bloodstream forms, and the genes upregulated included putative fatty acid β-oxidation enzymes. Consistent with this, ATFs were able to utilize exogenous myristate and form β-oxidation intermediates, suggesting that ATF parasites can use fatty acids as an external carbon source. These findings identify the adipose tissue as a niche for T. brucei during its mammalian life cycle and could potentially explain the weight loss associated with sleeping sickness.
Keywords: African trypanosomes; fat; fatty acid β-oxidation; metabolism; mouse infection; transcriptome.
Copyright © 2016 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
Parasite physiology: Trypanosomes chew the fat.Nat Rev Microbiol. 2016 Jul;14(7):402-3. doi: 10.1038/nrmicro.2016.86. Epub 2016 May 31. Nat Rev Microbiol. 2016. PMID: 27241044 No abstract available.
-
African Trypanosomes Find a Fat Haven.Cell Host Microbe. 2016 Jun 8;19(6):748-9. doi: 10.1016/j.chom.2016.05.022. Cell Host Microbe. 2016. PMID: 27281564 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

