Potential impact on blood availability and donor iron status of changes to donor hemoglobin cutoff and interdonation intervals
- PMID: 27237451
- PMCID: PMC4980222
- DOI: 10.1111/trf.13663
Potential impact on blood availability and donor iron status of changes to donor hemoglobin cutoff and interdonation intervals
Abstract
Background: A minimum male hemoglobin (Hb) level of 13.0 g/dL becomes a Food and Drug Administration requirement effective May 2016. In addition, extending whole blood (WB) interdonation intervals (IDIs) beyond 8 weeks has been considered to reduce iron depletion in repeat blood donors. This study estimates the impact these changes might have on blood availability and donor iron status.
Study design and methods: Six blood centers participating in Retrovirus Epidemiology Donor Study-II (REDS-II) collected information on all donation visits from 2006 to 2009. Simulations were developed from these data using a multistage approach that first sought to adequately reproduce the patterns of donor return, Hb and ferritin levels, and outcomes of a donor's visit (successful single- or double-red blood cell donation, deferral for low Hb) observed in REDS-II data sets. Modified simulations were used to predict the potential impact on the blood supply and donor iron status under different Hb cutoff and IDI qualification criteria.
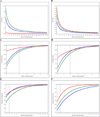
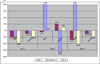
Results: More than 10% of WB donations might require replacement under many simulated scenarios. Longer IDIs would reduce the proportion of donors with iron depletion, but 80% of these donors may remain iron-depleted if minimal IDIs increased to 12 or 16 weeks.
Conclusion: Higher Hb cutoffs and longer IDIs are predicted to have a potentially large impact on collections but only a modest impact on donor iron depletion. Efforts to address iron depletion should be targeted to at-risk donors, such as iron supplementation programs for frequent donors, and policy makers should try to avoid broadly restrictive donation requirements that could substantially reduce blood availability.
© 2016 AABB.
Conflict of interest statement
Conflict of interest: The authors have no conflicts of interest relevant to this manuscript.
Figures

References
-
- AABB. The 2013 Blood Collection, Utilization, and Patient Blood Management Survey Report. Bethesda, MD: 2015.
-
- Federal Register. [cited January 17, 2016];80(99) Available from website: https://federalregister.gov/a/2015-12228.
-
- Custer B, Bravo M, Bruhn R, Land K, Tomasulo P, Kamel H. Predictors of hemoglobin recovery or deferral in blood donors with an initial successful donation. Transfusion. 2014;51(9):2267–2275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

