Ferric maltol therapy for iron deficiency anaemia in patients with inflammatory bowel disease: long-term extension data from a Phase 3 study
- PMID: 27237709
- PMCID: PMC5089582
- DOI: 10.1111/apt.13665
Ferric maltol therapy for iron deficiency anaemia in patients with inflammatory bowel disease: long-term extension data from a Phase 3 study
Abstract
Background: Ferric maltol was effective and well-tolerated in iron deficiency anaemia patients with inflammatory bowel disease during a 12-week placebo-controlled trial.
Aim: To perform a Phase 3 extension study evaluating long-term efficacy and safety with ferric maltol in inflammatory bowel disease patients in whom oral ferrous therapies had failed to correct iron deficiency anaemia.
Methods: After 12 weeks of randomised, double-blind treatment, patients with iron deficiency anaemia and mild-to-moderate ulcerative colitis or Crohn's disease received open-label ferric maltol 30 mg b.d. for 52 weeks.
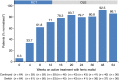
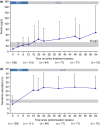
Results: 111 patients completed randomised treatment and 97 entered the open-label ferric maltol extension. In patients randomised to ferric maltol ('continued'; n = 50), mean ± s.d. haemoglobin increased by 3.07 ± 1.46 g/dL between baseline and Week 64. In patients randomised to placebo ('switch'; n = 47), haemoglobin increased by 2.19 ± 1.61 g/dL. Normal haemoglobin was achieved in high proportions of both continued and switch patients (89% and 83% at Week 64, respectively). Serum ferritin increased from 8.9 μg/L (baseline) to 26.0 μg/L (Week 12) in ferric maltol-treated patients, and to 57.4 μg/L amongst all patients at Week 64. In total, 80% of patients reported ≥1 adverse event by Week 64. Adverse events considered related to ferric maltol were recorded in 27/111 (24%) patients: 8/18 discontinuations due to adverse events were treatment-related. One patient was withdrawn due to increased ulcerative colitis activity.
Conclusions: Normal haemoglobin was observed in ≥80% of patients from weeks 20-64 of long-term ferric maltol treatment, with concomitant increases in iron storage parameters. Ferric maltol was well-tolerated throughout this 64-week study.
© 2016 The Authors. Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Figures

Comment in
-
Bedarf an i.v. Eisen sinkt um 70.MMW Fortschr Med. 2021 Sep;163(15):63. doi: 10.1007/s15006-021-0322-9. MMW Fortschr Med. 2021. PMID: 34478097 German. No abstract available.
References
-
- Breuer W, Hershko C, Cabantchik ZI. The importance of non‐transferrin bound iron in disorders of iron metabolism. Transfus Sci 2000; 23: 185–92. - PubMed
-
- Dignass AU, Gasche C, Bettenworth D, et al European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis 2015; 9: 211–22. - PubMed
-
- Stein J, Hartmann F, Dignass AU. Diagnosis and management of iron deficiency anemia in patients with IBD. Nat Rev Gastroenterol Hepatol 2010; 7: 599–610. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
