Sensory Neurons that Detect Stretch and Nutrients in the Digestive System
- PMID: 27238020
- PMCID: PMC4930427
- DOI: 10.1016/j.cell.2016.05.011
Sensory Neurons that Detect Stretch and Nutrients in the Digestive System
Erratum in
-
Sensory Neurons that Detect Stretch and Nutrients in the Digestive System.Cell. 2025 Jun 26;188(13):3623-3624. doi: 10.1016/j.cell.2025.05.043. Epub 2025 Jun 7. Cell. 2025. PMID: 40485186 Free PMC article. No abstract available.
Abstract
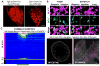
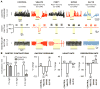
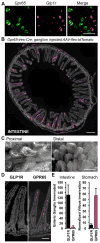
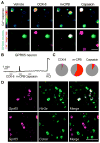
Neural inputs from internal organs are essential for normal autonomic function. The vagus nerve is a key body-brain connection that monitors the digestive, cardiovascular, and respiratory systems. Within the gastrointestinal tract, vagal sensory neurons detect gut hormones and organ distension. Here, we investigate the molecular diversity of vagal sensory neurons and their roles in sensing gastrointestinal inputs. Genetic approaches allowed targeted investigation of gut-to-brain afferents involved in homeostatic responses to ingested nutrients (GPR65 neurons) and mechanical distension of the stomach and intestine (GLP1R neurons). Optogenetics, in vivo ganglion imaging, and genetically guided anatomical mapping provide direct links between neuron identity, peripheral anatomy, central anatomy, conduction velocity, response properties in vitro and in vivo, and physiological function. These studies clarify the roles of vagal afferents in mediating particular gut hormone responses. Moreover, genetic control over gut-to-brain neurons provides a molecular framework for understanding neural control of gastrointestinal physiology.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Neurogastroenterology: A tale of two neurons - distinct functions of vagal afferents of the gut.Nat Rev Gastroenterol Hepatol. 2016 Aug;13(8):435. doi: 10.1038/nrgastro.2016.100. Epub 2016 Jun 15. Nat Rev Gastroenterol Hepatol. 2016. PMID: 27301540 No abstract available.
References
-
- Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain research. 2005;1044:127–131. - PubMed
-
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

