Pharmacological profile of Ascaris suum ACR-16, a new homomeric nicotinic acetylcholine receptor widely distributed in Ascaris tissues
- PMID: 27238203
- PMCID: PMC4959957
- DOI: 10.1111/bph.13524
Pharmacological profile of Ascaris suum ACR-16, a new homomeric nicotinic acetylcholine receptor widely distributed in Ascaris tissues
Abstract
Background and purpose: Control of nematode parasite infections relies largely on anthelmintic drugs, several of which act on nicotinic ACh receptors (nAChRs), and there are concerns about the development of resistance. There is an urgent need for development of new compounds to overcome resistance and novel anthelmintic drug targets. We describe the functional expression and pharmacological characterization of a homomeric nAChR, ACR-16, from a nematode parasite.
Experimental approach: Using RT-PCR, molecular cloning and two-electrode voltage clamp electrophysiology, we localized acr-16 mRNA in Ascaris suum (Asu) and then cloned and expressed acr-16 cRNA in Xenopus oocytes. Sensitivity of these receptors to cholinergic anthelmintics and a range of nicotinic agonists was tested.
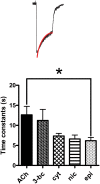
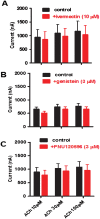
Key results: Amino acid sequence comparison with vertebrate nAChR subunits revealed ACR-16 to be most closely related to α7 receptors, but with some striking distinctions. acr-16 mRNA was recovered from Asu somatic muscle, pharynx, ovijector, head and intestine. In electrophysiological experiments, the existing cholinergic anthelmintic agonists (morantel, levamisole, methyridine, thenium, bephenium, tribendimidine and pyrantel) did not activate Asu-ACR-16 (except for a small response to oxantel). Other nAChR agonists: nicotine, ACh, cytisine, 3-bromocytisine and epibatidine, produced robust current responses which desensitized at a rate varying with the agonists. Unlike α7, Asu-ACR-16 was insensitive to α-bungarotoxin and did not respond to genistein or other α7 positive allosteric modulators. Asu-ACR-16 had lower calcium permeability than α7 receptors.
Conclusions and implications: We suggest that ACR-16 has diverse tissue-dependent functions in nematode parasites and is a suitable drug target for development of novel anthelmintic compounds.
© 2016 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Figures






References
-
- Ballivet M, Alliod C, Bertrand S, Bertrand D (1996). Nicotinic acetylcholine receptors in the nematode Caenorhabditis elegans . J Mol Biol 258: 261–269. - PubMed
-
- Baur R, Beech R, Sigel E, Rufener L (2015). Monepantel irreversibly binds to and opens Haemonchus contortus MPTL‐1 and Caenorhabditis elegans ACR‐20 receptors. Mol Pharmacol 87: 96–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

