Exosomes mediate hepatitis B virus (HBV) transmission and NK-cell dysfunction
- PMID: 27238466
- PMCID: PMC5423088
- DOI: 10.1038/cmi.2016.24
Exosomes mediate hepatitis B virus (HBV) transmission and NK-cell dysfunction
Abstract
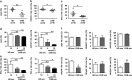
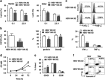
Evidence suggests that exosomes can transfer genetic material between cells. However, their roles in hepatitis B virus (HBV) infection remain unclear. Here, we report that exosomes present in the sera of chronic hepatitis B (CHB) patients contained both HBV nucleic acids and HBV proteins, and transferred HBV to hepatocytes in an active manner. Notably, HBV nucleic acids were detected in natural killer (NK) cells from both CHB patients and healthy donors after exposure to HBV-positive exosomes. Through real-time fluorescence microscopy and flow cytometry, 1,1'-dioctadecyl-3,3,3',3',-tetramethylindodicarbocyanine, 4-chlorobenzenesulfnate salt (DiD)-labeled exosomes were observed to interact with NK cells and to be taken up by NK cells, which was enhanced by transforming growth factor-β treatment. Furthermore, HBV-positive exosomes impaired NK-cell functions, including interferon (IFN)-γ production, cytolytic activity, NK-cell proliferation and survival, as well as the responsiveness of the cells to poly (I:C) stimulation. HBV infection suppressed the expression of pattern-recognition receptors, especially retinoic acid inducible gene I (RIG-I), on NK cells, resulting in the dampening of the nuclear factor κB(NF-κB) and p38 mitogen-activated protein kinase pathways. Our results highlight a previously unappreciated role of exosomes in HBV transmission and NK-cell dysfunction during CHB infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Comment in
-
Exosomes: multitask cargo carriers modulating innate immunity to viruses.Cell Mol Immunol. 2017 May;14(5):476-477. doi: 10.1038/cmi.2016.27. Epub 2016 Jun 6. Cell Mol Immunol. 2017. PMID: 27264688 Free PMC article. No abstract available.
Similar articles
-
Natural Killer p46 Controls Hepatitis B Virus Replication and Modulates Liver Inflammation.PLoS One. 2015 Aug 20;10(8):e0135874. doi: 10.1371/journal.pone.0135874. eCollection 2015. PLoS One. 2015. PMID: 26291078 Free PMC article.
-
T cell immunoglobulin- and mucin-domain-containing molecule-3 (Tim-3) mediates natural killer cell suppression in chronic hepatitis B.J Hepatol. 2010 Mar;52(3):322-9. doi: 10.1016/j.jhep.2009.12.005. Epub 2010 Jan 6. J Hepatol. 2010. PMID: 20133006
-
KLRG1+ natural killer cells exert a novel antifibrotic function in chronic hepatitis B.J Hepatol. 2019 Aug;71(2):252-264. doi: 10.1016/j.jhep.2019.03.012. Epub 2019 Mar 21. J Hepatol. 2019. PMID: 30905683
-
Natural killer cell dysfunction in hepatocellular carcinoma and NK cell-based immunotherapy.Acta Pharmacol Sin. 2015 Oct;36(10):1191-9. doi: 10.1038/aps.2015.41. Epub 2015 Jun 15. Acta Pharmacol Sin. 2015. PMID: 26073325 Free PMC article. Review.
-
The direct and indirect roles of HBV in liver cancer: prospective markers for HCC screening and potential therapeutic targets.J Pathol. 2015 Jan;235(2):355-67. doi: 10.1002/path.4434. J Pathol. 2015. PMID: 25196558 Review.
Cited by
-
Identification of the Level of Exosomal Protein by Parallel Reaction Monitoring Technology in HCC Patients.Int J Gen Med. 2022 Oct 14;15:7831-7842. doi: 10.2147/IJGM.S384140. eCollection 2022. Int J Gen Med. 2022. PMID: 36267426 Free PMC article.
-
Small extracellular vesicles as key players in cancer development caused by human oncogenic viruses.Infect Agent Cancer. 2022 Nov 28;17(1):58. doi: 10.1186/s13027-022-00471-x. Infect Agent Cancer. 2022. PMID: 36437456 Free PMC article. Review.
-
Turnip Mosaic Virus Components Are Released into the Extracellular Space by Vesicles in Infected Leaves.Plant Physiol. 2019 Jul;180(3):1375-1388. doi: 10.1104/pp.19.00381. Epub 2019 Apr 24. Plant Physiol. 2019. PMID: 31019004 Free PMC article.
-
The Dynamic Role of NK Cells in Liver Cancers: Role in HCC and HBV Associated HCC and Its Therapeutic Implications.Front Immunol. 2022 May 20;13:887186. doi: 10.3389/fimmu.2022.887186. eCollection 2022. Front Immunol. 2022. PMID: 35669776 Free PMC article. Review.
-
Exosome and virus infection.Front Immunol. 2023 Mar 30;14:1154217. doi: 10.3389/fimmu.2023.1154217. eCollection 2023. Front Immunol. 2023. PMID: 37063897 Free PMC article. Review.
References
-
- Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat 2004; 11: 97–107. - PubMed
-
- McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology 2009; 49: S45–S55. - PubMed
-
- McMahon BJ. Chronic hepatitis B virus infection. Med Clin North Am 2014; 98: 39–54. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

