Induction of HEXIM1 activities by HMBA derivative 4a1: Functional consequences and mechanism
- PMID: 27238569
- PMCID: PMC4988685
- DOI: 10.1016/j.canlet.2016.05.029
Induction of HEXIM1 activities by HMBA derivative 4a1: Functional consequences and mechanism
Abstract
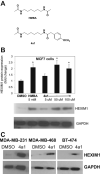
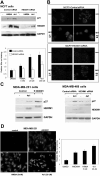
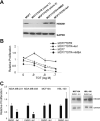
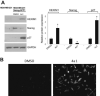
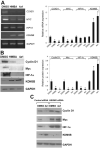
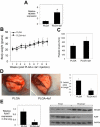
We have been studying the role of Hexamethylene bisacetamide (HMBA) Induced Protein 1 (HEXIM1) as a tumor suppressor whose expression is decreased in tamoxifen resistant and metastatic breast cancer. HMBA was considered the most potent and specific inducer for HMBA inducible protein 1 (HEXIM1) prior to our studies. Moreover, the ability of HMBA to induce differentiation is advantageous for its therapeutic use when compared to cytotoxic agents. However, HMBA induced HEXIM1 expression required at mM concentrations and induced dose limiting toxicity, thrombocytopenia. Thus we structurally optimized HMBA and identified a more potent inducer of HEXIM1 expression, 4a1. The studies reported herein tested the ability of 4a1 to induce HEXIM1 activities using a combination of biochemical, cell phenotypic, and in vivo assays. 4a1 induced breast cell differentiation, including the stem cell fraction in triple negative breast cancer cells. Clinically relevant HEXIM1 activities that are also induced by 4a1 include enhancement of the inhibitory effects of tamoxifen and inhibition of breast tumor metastasis. We also provide mechanistic basis for the phenotypic effects of 4a1. Our results support the potential of an unsymmetrical HMBA derivative, such as 4a1, as lead compound for further drug development.
Keywords: Antiestrogens; Breast cells; Differentiation; HEXIM1; HMBA; Metastasis.
Copyright © 2016 Elsevier Ireland Ltd. All rights reserved.
Figures

References
-
- Andreeff M, Stone R, Michaeli J, Young CW, Tong WP, Sogoloff H, Ervin T, Kufe D, Rifkind RA, Marks PA. Hexamethylene bisacetamide in myelodysplastic syndrome and acute myelogenous leukemia: a phase II clinical trial with a differentiation-inducing agent. Blood. 1992;80:2604–2609. - PubMed
-
- Young CW, Fanucchi MP, Declan Walsh T, Baltzer L, Yaldaei S, Stevens YW, Gordon C, Tong W, Rifkind RA, Marks PA. Phase I trial and clinical pharmacological evaluation of hexamethylene bisacetamide administration by ten-day continuous intravenous infusion at twenty-eight-day intervals. Cancer Res. 1988;48:7304–7309. - PubMed
-
- Turano M, Napolitano G, Dulac C, Majello B, Bensaude O, Lania L. Increased HEXIM1 expression during erythroleukemia and neuroblastoma cell differentiation. J Cell Physiol. 2006;206:603–610. - PubMed
-
- Conley BA, Forrest A, Egorin MJ, Zuhowski EG, Sinibaldi V, Van Echo DA. Phase I trial using adaptive control dosing of hexamethylene bisacetamide (NSC 95580). Cancer Res. 1989;49:3436–3440. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

