Neuropilin-1highCD4⁺CD25⁺ Regulatory T Cells Exhibit Primary Negative Immunoregulation in Sepsis
- PMID: 27239104
- PMCID: PMC4863118
- DOI: 10.1155/2016/7132158
Neuropilin-1highCD4⁺CD25⁺ Regulatory T Cells Exhibit Primary Negative Immunoregulation in Sepsis
Retraction in
-
Retracted: Neuropilin-1highCD4+CD25+ Regulatory T Cells Exhibit Primary Negative Immunoregulation in Sepsis.Mediators Inflamm. 2020 Sep 18;2020:9195623. doi: 10.1155/2020/9195623. eCollection 2020. Mediators Inflamm. 2020. PMID: 33013199 Free PMC article.
Abstract
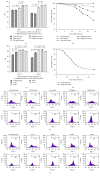
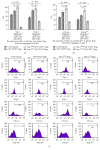
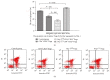
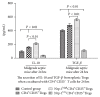
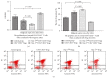
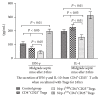
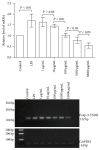
Regulatory T cells (Tregs) appear to be involved in sepsis-induced immune dysfunction; neuropilin-1 (Nrp-1) was identified as a surface marker for CD4(+)CD25(+)Tregs. In the current study, we investigated the negative immunoregulation of Nrp-1(high)CD4(+)CD25(+)Tregs and the potential therapeutic value of Nrp-1 in sepsis. Splenic CD4(+)CD25(+)Tregs from cecal ligation and puncture (CLP) mouse models were further segregated into Nrp-1(high)Tregs and Nrp-1(low)Tregs; they were cocultured with CD4(+)CD25(-) T cells. The expression of forkhead/winged helix transcription factor-3 (Foxp-3), cytotoxic T-lymphocyte associated antigen-4 (CTLA-4), membrane associated transforming growth factor-β (TGF-β(m+)), apoptotic rate, and secretive ability [including TGF-β and interleukin-10 (IL-10)] for various types of Tregs, as well as the immunosuppressive ability of Tregs on CD4(+)CD25(-) T cells, were determined. Meanwhile, the impact of recombinant Nrp-1 polyclonal antibody on the demethylation of Foxp-3-TSDR (Treg-specific demethylated region) was measured in in vitro study. Sepsis per se markedly promoted the expression of Nrp-1 of CD4(+)CD25(+)Tregs. Foxp-3/CTLA-4/TGF-β(m+) of Nrp-1(high)Tregs were upregulated by septic challenge. Nrp-1(high)Tregs showed strong resilience to apoptosis and secretive ability and the strongest immunosuppressive ability on CD4(+)CD25(-) T cells. In the presence of lipopolysaccharide (LPS), the recombinant Nrp-1 polyclonal antibody reduced the demethylation of Foxp-3-TSDR. Nrp-1(high)Tregs might reveal primary negative immunoregulation in sepsis; Nrp-1 could represent a new potential therapeutic target for the study of immune regulation in sepsis.
Figures
Similar articles
-
Tuftsin prevents the negative immunoregulation of neuropilin-1highCD4+CD25+Regulatory T cells and improves survival rate in septic mice.Oncotarget. 2016 Dec 6;7(49):81791-81805. doi: 10.18632/oncotarget.13235. Oncotarget. 2016. PMID: 27835904 Free PMC article.
-
Targeting Neuropilin-1 Suppresses the Stability of CD4+ CD25+ Regulatory T Cells via the NF-κB Signaling Pathway in Sepsis.Infect Immun. 2021 Jan 19;89(2):e00399-20. doi: 10.1128/IAI.00399-20. Print 2021 Jan 19. Infect Immun. 2021. PMID: 33139385 Free PMC article.
-
Effect of Regulatory T Cells on Promoting Apoptosis of T Lymphocyte and Its Regulatory Mechanism in Sepsis.J Interferon Cytokine Res. 2015 Dec;35(12):969-80. doi: 10.1089/jir.2014.0235. Epub 2015 Aug 26. J Interferon Cytokine Res. 2015. PMID: 26309018 Free PMC article.
-
The characterization and role of regulatory T cells in immune reactions.Front Biosci. 2008 Jan 1;13:2266-74. doi: 10.2741/2840. Front Biosci. 2008. PMID: 17981708 Review.
-
Surveillance of antigen-presenting cells by CD4+ CD25+ regulatory T cells in autoimmunity: immunopathogenesis and therapeutic implications.Am J Pathol. 2009 May;174(5):1575-87. doi: 10.2353/ajpath.2009.080987. Epub 2009 Apr 6. Am J Pathol. 2009. PMID: 19349365 Free PMC article. Review.
Cited by
-
CD4+Foxp3+T Regulatory Cells Promote Transplantation Tolerance by Modulating Effector CD4+ T Cells in a Neuropilin-1-Dependent Manner.Front Immunol. 2019 Apr 24;10:882. doi: 10.3389/fimmu.2019.00882. eCollection 2019. Front Immunol. 2019. PMID: 31068948 Free PMC article.
-
Correlation analysis of omega-3 fatty acids and mortality of sepsis and sepsis-induced ARDS in adults: data from previous randomized controlled trials.Nutr J. 2018 May 31;17(1):57. doi: 10.1186/s12937-018-0356-8. Nutr J. 2018. PMID: 29859104 Free PMC article. Review.
-
Hydroxysafflor Yellow A Attenuates the Apoptosis of Peripheral Blood CD4+ T Lymphocytes in a Murine Model of Sepsis.Front Pharmacol. 2017 Sep 6;8:613. doi: 10.3389/fphar.2017.00613. eCollection 2017. Front Pharmacol. 2017. PMID: 28932195 Free PMC article.
-
Histone Deacetylation Inhibitors as Modulators of Regulatory T Cells.Int J Mol Sci. 2020 Mar 29;21(7):2356. doi: 10.3390/ijms21072356. Int J Mol Sci. 2020. PMID: 32235291 Free PMC article. Review.
-
Th17/Treg imbalance in patients with severe acute pancreatitis: Attenuated by high-volume hemofiltration treatment.Medicine (Baltimore). 2020 Jul 31;99(31):e21491. doi: 10.1097/MD.0000000000021491. Medicine (Baltimore). 2020. PMID: 32756180 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

