Cognitive-Enhancing Effect of Aronia melanocarpa Extract against Memory Impairment Induced by Scopolamine in Mice
- PMID: 27239211
- PMCID: PMC4867058
- DOI: 10.1155/2016/6145926
Cognitive-Enhancing Effect of Aronia melanocarpa Extract against Memory Impairment Induced by Scopolamine in Mice
Abstract
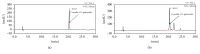
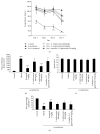
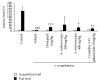
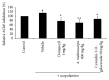
Aronia melanocarpa (A. melanocarpa) berries are a fruit with a marked antioxidant effect. The objective of this study was to confirm the effect of A. melanocarpa berries extract against scopolamine-induced memory impairment in mice using the Morris water maze and passive avoidance test. Moreover, we determined a possible mechanism of the cognitive-enhancing effect involving AChE activity and BDNF and p-CREB expression in the hippocampus of mice. A. melanocarpa berries extract attenuated the learning and memory impairment induced by scopolamine in the Morris water maze (79.3 ± 0.8 s of 200 mg/kg and 64.4 ± 10.7 s of 400 mg/kg on day 4) and passive avoidance tests (46.0 ± 41.1 s of 200 mg/kg and 25.6 ± 18.7 s of 400 mg/kg). A. melanocarpa berries extract reduced the acetylcholinesterase level in the hippocampus of scopolamine-injected mice and increased BDNF and p-CREB expression in the hippocampus. The major compound, cyanidin-3-O-galactoside, also reversed memory impairment. These results showed that A. melanocarpa berries extract improved memory impairment by inhibiting AChE and increasing BDNF and p-CREB expression, and cyanidin-3-O-galactoside may be responsible for the effect of A. melanocarpa berries extract.
Figures
Similar articles
-
Casticin ameliorates scopolamine-induced cognitive dysfunction in mice.J Ethnopharmacol. 2020 Sep 15;259:112843. doi: 10.1016/j.jep.2020.112843. Epub 2020 May 4. J Ethnopharmacol. 2020. PMID: 32380246
-
N-palmitoyl serotonin alleviates scopolamine-induced memory impairment via regulation of cholinergic and antioxidant systems, and expression of BDNF and p-CREB in mice.Chem Biol Interact. 2015 Dec 5;242:153-62. doi: 10.1016/j.cbi.2015.09.016. Epub 2015 Sep 25. Chem Biol Interact. 2015. PMID: 26408985
-
Z-Guggulsterone Improves the Scopolamine-Induced Memory Impairments Through Enhancement of the BDNF Signal in C57BL/6J Mice.Neurochem Res. 2016 Dec;41(12):3322-3332. doi: 10.1007/s11064-016-2064-0. Epub 2016 Sep 28. Neurochem Res. 2016. PMID: 27677871
-
Cognitive-Enhancing Effect of Dianthus superbus var. Longicalycinus on Scopolamine-Induced Memory Impairment in Mice.Biomol Ther (Seoul). 2016 May 1;24(3):298-304. doi: 10.4062/biomolther.2015.083. Biomol Ther (Seoul). 2016. PMID: 27133261 Free PMC article.
-
Antidiabetic Effects of Aronia melanocarpa and Its Other Therapeutic Properties.Front Nutr. 2017 Nov 6;4:53. doi: 10.3389/fnut.2017.00053. eCollection 2017. Front Nutr. 2017. PMID: 29164127 Free PMC article. Review.
Cited by
-
In Vivo Cognitive-Enhancing, Ex Vivo Malondialdehyde-Lowering Activities and Phytochemical Profiles of Aqueous and Methanolic Stem Bark Extracts of Piliostigma thonningii (Schum.).Int J Alzheimers Dis. 2020 Mar 24;2020:1367075. doi: 10.1155/2020/1367075. eCollection 2020. Int J Alzheimers Dis. 2020. PMID: 32308992 Free PMC article.
-
Cyanidin 3-O-galactoside: A Natural Compound with Multiple Health Benefits.Int J Mol Sci. 2021 Feb 24;22(5):2261. doi: 10.3390/ijms22052261. Int J Mol Sci. 2021. PMID: 33668383 Free PMC article. Review.
-
Src Tyrosine Kinase Inhibitory and Antioxidant Activity of Black Chokeberry and Bilberry Fruit Extracts Rich in Chlorogenic Acid.Int J Mol Sci. 2023 Oct 24;24(21):15512. doi: 10.3390/ijms242115512. Int J Mol Sci. 2023. PMID: 37958496 Free PMC article.
-
The Effect of Long-Term Aronia melanocarpa Extract Supplementation on Cognitive Performance, Mood, and Vascular Function: A Randomized Controlled Trial in Healthy, Middle-Aged Individuals.Nutrients. 2020 Aug 17;12(8):2475. doi: 10.3390/nu12082475. Nutrients. 2020. PMID: 32824483 Free PMC article. Clinical Trial.
-
Preclinical Evidence of Curcuma longa and Its Noncurcuminoid Constituents against Hepatobiliary Diseases: A Review.Evid Based Complement Alternat Med. 2020 Jul 28;2020:8761435. doi: 10.1155/2020/8761435. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 32802138 Free PMC article. Review.
References
-
- Crapper D. R., DeBoni U. Brain aging and Alzheimer's disease. Canadian Psychiatric Association Journal. 1978;23(4):229–233. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

