Du-Huo-Ji-Sheng-Tang Attenuates Inflammation of TNF-Tg Mice Related to Promoting Lymphatic Drainage Function
- PMID: 27239212
- PMCID: PMC4863122
- DOI: 10.1155/2016/7067691
Du-Huo-Ji-Sheng-Tang Attenuates Inflammation of TNF-Tg Mice Related to Promoting Lymphatic Drainage Function
Abstract
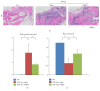
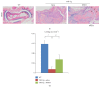
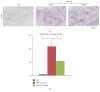
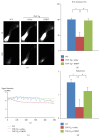
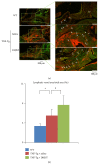
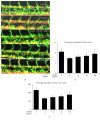
To investigate whether Du-Huo-Ji-Sheng-Tang (DHJST) attenuate inflammation of RA related to lymphatic drainage function in vivo, we treated eight 3-month-old TNF-Tg mice with DHJST (12 g/kg) or the same volume of physiological saline once every day for 12 weeks, and 3-month-old WT littermates were used as negative control. After twelve weeks, we performed NIR-ICG imaging and found that DHJST increased the ICG clearance at the footpad and the pulse of efferent lymphatic vessel between popliteal lymph node and footpad. Histology staining at ankle joints showed that DHJST decreases synovial inflammation, bone erosion, cartilage erosion, and TRAP+ osteoclast area in TNF-Tg mice. Immunohistochemical staining by using anti-Lyve-1 and anti-podoplanin antibody showed that DHJST stimulated lymphangiogenesis in ankle joints of TNF-Tg mice. And zebrafish study suggested that DHJST promoted the formation of lymphatic thoracic duct. In conclusion, DHJST inhibits inflammation severity and promotes lymphangiogenesis and lymphatic drainage function of TNF-Tg mice.
Figures
References
-
- Olszewski W. L., Pazdur J., Kubasiewicz E., Zaleska M., Cooke C. J., Miller N. E. Lymph draining from foot joints in rheumatoid arthritis provides insight into local cytokine and chemokine production and transport to lymph nodes. Arthritis and Rheumatism. 2001;44(3):541–549. - PubMed
-
- Wauke K., Nagashima M., Ishiwata T., Asano G., Yoshino S. Expression and localization of vascular endothelial growth factor-C in rheumatoid arthritis synovial tissue. The Journal of Rheumatology. 2002;29(1):34–38. - PubMed
-
- Zhou Q., Wood R., Schwarz E. M., Wang Y.-J., Xing L. Near-infrared lymphatic imaging demonstrates the dynamics of lymph flow and lymphangiogenesis during the acute versus chronic phases of arthritis in mice. Arthritis and Rheumatism. 2010;62(7):1881–1889. doi: 10.1002/art.27464. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

