Ramucirumab in metastatic colorectal cancer: evidence to date and place in therapy
- PMID: 27239240
- PMCID: PMC4872251
- DOI: 10.1177/1758834016635888
Ramucirumab in metastatic colorectal cancer: evidence to date and place in therapy
Abstract
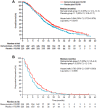
Colorectal cancer is the third most frequent cancer worldwide. Overall survival rates have improved greatly over the last few years due, at least in part, to the addition of targeted therapies to standard of care chemotherapy. Angiogenesis plays an important role in colorectal cancer, and therapies directed against the vascular endothelial growth factor (VEGF) axis have contributed significantly to improving the outcome of patients with metastatic colorectal cancer. Over the past few years, several new targeted antiangiogenic agents have been approved for this patient population, confirming the value of inhibiting tumour angiogenesis. The most recent among them is ramucirumab, a fully humanized monoclonal antibody that targets the extracellular domain of VEGF receptor 2. It has proven valuable in multiple tumour types including colorectal cancer. Several phase I and II clinical trials showed a favourable toxicity profile and promising clinical antitumour efficacy in colorectal cancer patients. In the phase III RAISE clinical trial, the addition of ramucirumab to FOLFIRI-based chemotherapy resulted in an improvement of overall survival in patients with metastatic colorectal cancer who had been previously treated with bevacizumab, oxaliplatin and a fluoropyrimidine. On the basis of these results, ramucirumab was approved by the US Food and Drug Administration for this setting. We present an overview of the key preclinical and clinical studies in the development of ramucirumab in the context of metastatic colorectal cancer.
Keywords: angiogenesis; colorectal cancer; metastatic; ramucirumab; vascular endothelial growth factor.
Conflict of interest statement
Figures


References
-
- Bennouna J., Sastre J., Arnold D., Österlund P., Greil R., Van Cutsem E., et al. (2013) Continuation of bevacizumab after first progression in metastatic colorectal cancer (Ml18147): a randomised phase III trial. Lancet Oncol 14: 29–37. - PubMed
-
- Bruns C., Liu W., Davis D., Shaheen R., McConkey D., Wilson M., et al. (2000) Vascular endothelial growth factor is an in vivo survival factor for tumor endothelium in a murine model of colorectal carcinoma liver metastases. Cancer 89: 488–499. - PubMed
-
- Bruns C., Shrader M., Harbison M., Portera C., Solorzano C., Jauch K., et al. (2002) Effect of the vascular endothelial growth factor receptor-2 antibody DC101 plus gemcitabine on growth, metastasis and angiogenesis of human pancreatic cancer growing orthotopically in nude mice. Int J Cancer 102: 101–108. - PubMed
-
- Chiorean E., Hurwitz H., Cohen R., Schwartz J., Dalal R., Fox F., et al. (2015) Phase I study of every 2- or 3-week dosing of ramucirumab, a human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2 in patients with advanced solid tumors. Ann Oncol 26: 1230–1237. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

