Singlet Oxygen Generation by Laser Irradiation of Gold Nanoparticles
- PMID: 27239247
- PMCID: PMC4878812
- DOI: 10.1021/acs.jpcc.6b02005
Singlet Oxygen Generation by Laser Irradiation of Gold Nanoparticles
Abstract
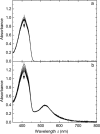
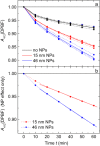
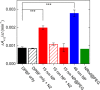
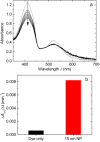
The formation of singlet oxygen by irradiation of gold nanoparticles in their plasmon resonance band with continuous or pulsed laser light has been investigated. Citrate-stabilized nanoparticles were found to facilitate the photogeneration of singlet oxygen, albeit with low quantum yield. The reaction caused by pulsed laser irradiation makes use of the equilibrated hot electrons that can reach temperatures of several thousand degrees during the laser pulse. Although less efficient, continuous irradiation, which acts via the short-lived directly excited primary "hot" electrons only, can produce enough singlet oxygen for photodynamic cancer therapy and has significant advantages for practical applications. However, careful design of the nanoparticles is needed, since even a moderately thick capping layer can completely inhibit singlet oxygen formation. Moreover, the efficiency of the process also depends on the nanoparticle size.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
