Effect of the specific proteasome inhibitor bortezomib on cancer-related muscle wasting
- PMID: 27239411
- PMCID: PMC4864285
- DOI: 10.1002/jcsm.12050
Effect of the specific proteasome inhibitor bortezomib on cancer-related muscle wasting
Abstract
Background: Muscle wasting, a prominent feature of cancer cachexia, is mainly caused by sustained protein hypercatabolism. The enhanced muscle protein degradation rates rely on the activity of different proteolytic systems, although the Adenosine triphosphate (ATP)-ubiquitin-proteasome-dependent pathway and autophagy have been shown to play a pivotal role. Bortezomib is a potent reversible and selective proteasome and NF-κB inhibitor approved for the clinical use, which has been shown to be effective in preventing muscle wasting in different catabolic conditions. The aim of the present study has been to investigate whether pharmacological inhibition of proteasome by bortezomib may prevent skeletal muscle wasting in experimental cancer cachexia.
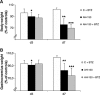
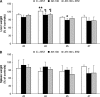
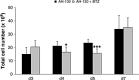
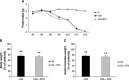
Methods: Cancer cachexia was induced in rats by intraperitoneal injection of Yoshida AH-130 ascites hepatoma cells and in mice by subcutaneous inoculation of C26 carcinoma cells. Animals were then further randomized to receive bortezomib. The AH-130 hosts were weighted and sacrificed under anaesthesia, on Days 3, 4, 5, and 7 after tumour inoculation, while C26-bearing mice were weighted and sacrificed under anaesthesia 12 days after tumour transplantation. NF-κB and proteasome activation, MuRF1 and atrogin-1 mRNA expression and beclin-1 protein levels were evaluated in the gastrocnemius of controls and AH-130 hosts.
Results: Bortezomib administration in the AH-130 hosts, although able to reduce proteasome and NF-κB DNA-binding activity in the skeletal muscle on Day 7 after tumour transplantation, did not prevent body weight loss and muscle wasting. In addition, bortezomib exerted a transient toxicity, as evidenced by the reduced food intake and by the increase in NF-κB DNA-binding activity in the AH-130 hosts 3 days after tumour transplantation. Beclin-1 protein levels were increased by bortezomib treatment in Day 3 controls but were unchanged on both Days 3 and 7 in the AH-130 hosts, suggesting that an early compensatory induction of autophagy may exist in healthy but not in tumour-bearing animals. Regarding C26-bearing mice, bortezomib did not prevent as well body and muscle weight loss 12 days after tumour implantation.
Conclusions: The results obtained suggest that proteasome inhibition by bortezomib is not able to prevent muscle wasting in experimental cancer cachexia. Further studies are needed to address the issue whether a different dosage of bortezomib alone or in combination with other drugs modulating different molecular pathways may effectively prevent muscle wasting during cancer cachexia.
Keywords: Bortezomib; Cancer cachexia; Muscle wasting; Proteasome.
Figures




Similar articles
-
Interference with Ca2+-Dependent Proteolysis Does Not Alter the Course of Muscle Wasting in Experimental Cancer Cachexia.Front Physiol. 2017 Apr 19;8:213. doi: 10.3389/fphys.2017.00213. eCollection 2017. Front Physiol. 2017. PMID: 28469577 Free PMC article.
-
IGF-1 is downregulated in experimental cancer cachexia.Am J Physiol Regul Integr Comp Physiol. 2006 Sep;291(3):R674-83. doi: 10.1152/ajpregu.00104.2006. Epub 2006 Apr 13. Am J Physiol Regul Integr Comp Physiol. 2006. PMID: 16614058
-
Megestrol acetate improves cardiac function in a model of cancer cachexia-induced cardiomyopathy by autophagic modulation.J Cachexia Sarcopenia Muscle. 2016 Dec;7(5):555-566. doi: 10.1002/jcsm.12116. Epub 2016 Apr 7. J Cachexia Sarcopenia Muscle. 2016. PMID: 27239419 Free PMC article.
-
The ubiquitin-proteasome pathway as a therapeutic target for muscle wasting.J Support Oncol. 2005 May-Jun;3(3):209-17. J Support Oncol. 2005. PMID: 15915823 Review.
-
Muscle wasting in cancer.Int J Biochem Cell Biol. 2013 Oct;45(10):2215-29. doi: 10.1016/j.biocel.2013.05.032. Epub 2013 Jun 11. Int J Biochem Cell Biol. 2013. PMID: 23770121 Review.
Cited by
-
Sex-specific role of myostatin signaling in neonatal muscle growth, denervation atrophy, and neuromuscular contractures.Elife. 2022 Oct 31;11:e81121. doi: 10.7554/eLife.81121. Elife. 2022. PMID: 36314781 Free PMC article.
-
Characterization of cachexia in the human fibrosarcoma HT-1080 mouse tumour model.J Cachexia Sarcopenia Muscle. 2020 Dec;11(6):1813-1829. doi: 10.1002/jcsm.12618. Epub 2020 Sep 13. J Cachexia Sarcopenia Muscle. 2020. PMID: 32924335 Free PMC article.
-
Machine learning-based phenotypic screening for postmitotic growth inducers uncover vitamin D3 metabolites as small molecule ribosome agonists.Cell Prolif. 2022 May;55(5):e13214. doi: 10.1111/cpr.13214. Epub 2022 Apr 12. Cell Prolif. 2022. PMID: 35411556 Free PMC article.
-
Time to jump on the bandwagon: the Journal of Cachexia, Sarcopenia and Muscle in 2018.J Cachexia Sarcopenia Muscle. 2018 Oct;9(5):793-801. doi: 10.1002/jcsm.12356. Epub 2018 Oct 11. J Cachexia Sarcopenia Muscle. 2018. PMID: 30311438 Free PMC article. No abstract available.
-
Towards Drug Repurposing in Cancer Cachexia: Potential Targets and Candidates.Pharmaceuticals (Basel). 2021 Oct 26;14(11):1084. doi: 10.3390/ph14111084. Pharmaceuticals (Basel). 2021. PMID: 34832866 Free PMC article. Review.
References
-
- Muscaritoli M, Molfino A, Gioia G, Laviano A, Rossi Fanelli F. The “parallel pathway”: a novel nutritional and metabolic approach to cancer patients. Intern Emerg Med 2011;6:105–12. - PubMed
-
- Muscaritoli M, Bossola M, Aversa Z, Bellantone R, Rossi Fanelli F. Prevention and treatment of cancer cachexia: new insight into an old problem. Eur J Cancer 2006;42:31–41. - PubMed
-
- Acharyya S, Guttridge DC. Cancer cachexia signaling pathways continue to emerge yet much still points to the proteasome. Clin Cancer Res 2007;13:1356–61. - PubMed
-
- Penna F, Baccino FM, Costelli P. Coming back: autophagy in cachexia. Curr Opin Clin Nutr Metab Care 2014;17:241–6. - PubMed
-
- Ciechanover A. The ubiquitin‐proteasome proteolytic pathway. Cell 1994;79:13–21. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

