Amyloid-related imaging abnormalities from trials of solanezumab for Alzheimer's disease
- PMID: 27239538
- PMCID: PMC4879647
- DOI: 10.1016/j.dadm.2016.02.004
Amyloid-related imaging abnormalities from trials of solanezumab for Alzheimer's disease
Abstract
Introduction: Solanezumab, a humanized monoclonal antibody that binds soluble amyloid beta peptide, is being developed for treatment of Alzheimer's disease (AD).
Methods: Patients (n = 2042) with mild and moderate AD were randomized 1:1 to 400-mg solanezumab or placebo infusion every 4 weeks for 80 weeks and 1457 patients entered an open-label extension. Magnetic resonance imaging scans monitored for amyloid-related imaging abnormalities-edema/effusion (ARIA-E) and amyloid-related imaging abnormalities-hemorrhage/hemosiderin deposition.
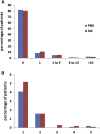
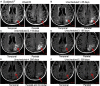
Results: Sixteen patients (solanezumab, n = 11; placebo, n = 5) developed ARIA-E during the double-blind phase, and 7 patients developed ARIA-E during the open-label extension as of July 31, 2014. Unique cases are discussed including solanezumab patients who were given solanezumab, while ARIA-E was present and a patient who developed ARIA-E during placebo treatment and again during solanezumab treatment.
Discussion: Asymptomatic ARIA-E was detected in solanezumab-treated and placebo-treated AD patients. ARIA-E occurs infrequently during solanezumab and placebo treatments but may occur repeatedly in some patients.
Keywords: Alzheimer's disease; Amyloid-related imaging abnormalities; Clinical trials.
Figures



References
-
- Carlson C., Estergard W., Oh J., Suhy J., Jack C.R., Siemers E. Prevalence of asymptomatic vasogenic edema in pretreatment Alzheimer's disease study cohorts from phase 3 trials of semagacestat and solanezumab. Alzheimers Dement. 2011;7:396–401. - PubMed
-
- Goos J.D., Henneman W.J., Sluimer J.D., Vrenken H., Sluimer I.C., Barkhof F. Incidence of cerebral microbleeds: A longitudinal study in a memory clinic population. Neurology. 2010;74:1954–1960. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
