Adjuvant-dependent innate and adaptive immune signatures of risk of SIVmac251 acquisition
- PMID: 27239761
- PMCID: PMC5916782
- DOI: 10.1038/nm.4105
Adjuvant-dependent innate and adaptive immune signatures of risk of SIVmac251 acquisition
Erratum in
-
Corrigendum: Adjuvant-dependent innate and adaptive immune signatures of risk of SIVmac251 acquisition.Nat Med. 2016 Oct 6;22(10):1192. doi: 10.1038/nm1016-1192a. Nat Med. 2016. PMID: 27711066 No abstract available.
Abstract
A recombinant vaccine containing Aventis Pasteur's canarypox vector (ALVAC)-HIV and gp120 alum decreased the risk of HIV acquisition in the RV144 vaccine trial. The substitution of alum with the more immunogenic MF59 adjuvant is under consideration for the next efficacy human trial. We found here that an ALVAC-simian immunodeficiency virus (SIV) and gp120 alum (ALVAC-SIV + gp120) equivalent vaccine, but not an ALVAC-SIV + gp120 MF59 vaccine, was efficacious in delaying the onset of SIVmac251 in rhesus macaques, despite the higher immunogenicity of the latter adjuvant. Vaccine efficacy was associated with alum-induced, but not with MF59-induced, envelope (Env)-dependent mucosal innate lymphoid cells (ILCs) that produce interleukin (IL)-17, as well as with mucosal IgG to the gp120 variable region 2 (V2) and the expression of 12 genes, ten of which are part of the RAS pathway. The association between RAS activation and vaccine efficacy was also observed in an independent efficacious SIV-vaccine approach. Whether RAS activation, mucosal ILCs and antibodies to V2 are also important hallmarks of HIV-vaccine efficacy in humans will require further studies.
Figures
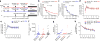


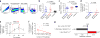


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

