The actions of relaxin on the human cardiovascular system
- PMID: 27239943
- PMCID: PMC5406304
- DOI: 10.1111/bph.13523
The actions of relaxin on the human cardiovascular system
Erratum in
-
Correction.Br J Pharmacol. 2017 Dec;174(24):4836. doi: 10.1111/bph.14111. Br J Pharmacol. 2017. PMID: 29235105 Free PMC article. No abstract available.
Abstract
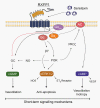
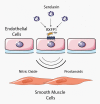
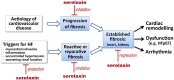
The insulin-like peptide relaxin, originally identified as a hormone of pregnancy, is now known to exert a range of pleiotropic effects including vasodilatory, anti-fibrotic, angiogenic, anti-apoptotic and anti-inflammatory effects in both males and females. Relaxin produces these effects by binding to a cognate receptor RXFP1 and activating a variety of signalling pathways including cAMP, cGMP and MAPKs as well as by altering gene expression of TGF-β, MMPs, angiogenic growth factors and endothelin receptors. The peptide has been shown to be effective in halting or reversing many of the adverse effects including fibrosis in animal models of cardiovascular disease including ischaemia/reperfusion injury, myocardial infarction, hypertensive heart disease and cardiomyopathy. Relaxin given to humans is safe and produces favourable haemodynamic changes. Serelaxin, the recombinant form of relaxin, is now in extended phase III clinical trials for the treatment of acute heart failure. Previous clinical studies indicated that a 48 h infusion of relaxin improved 180 day mortality, yet the mechanism underlying this effect is not clear. This article provides an overview of the cellular mechanism of effects of relaxin and summarizes its beneficial actions in animal models and in the clinic. We also hypothesize potential mechanisms for the clinical efficacy of relaxin, identify current knowledge gaps and suggest new ways in which relaxin could be useful therapeutically.
Linked articles: This article is part of a themed section on Recent Progress in the Understanding of Relaxin Family Peptides and their Receptors. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.10/issuetoc.
© 2016 The British Pharmacological Society.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

