The optimal time for surgery in women with serous ovarian cancer
- PMID: 27240134
- PMCID: PMC4961484
- DOI: 10.1503/cjs.014315
The optimal time for surgery in women with serous ovarian cancer
Erratum in
-
Correction: The optimal time for surgery in women with serous ovarian cancer.Can J Surg. 2016 Sep;59(5):295. Can J Surg. 2016. PMID: 27668327 Free PMC article. No abstract available.
Abstract
Background: Advanced high-grade serous ovarian carcinoma (HGSC) is commonly treated with surgery and chemotherapy. We investigated the survival of patients treated with primary or interval surgery at different times following neoadjuvant chemotherapy. Their survival was compared with that of patients treated with primary cytoreductive surgery and adjuvant chemotherapy.
Methods: Patients with stage III or IV HGSC were included in this retrospective cohort study. Clinical data were obtained from patient records. Patients were divided into 2 groups based on treatment with neoadjuvant chemotherapy and interval cytoreductive surgery (NAC) or with primary cytoreductive surgery and adjuvant chemotherapy (PCS). Study groups were stratified by several clinical variables.
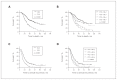
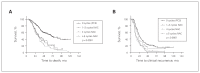
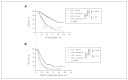
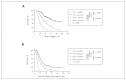
Results: We included 334 patients in our study: 156 in the NAC and 178 in the PCS groups. Survival of patients in the NAC group was independent of when they underwent interval cytoreductive surgery following initiation of neoadjuvant chemotherapy (p < 0.001). Optimal surgical cytoreduction had no impact on overall survival in the NAC group (p < 0.001). Optimal cytoreduction (p < 0.001) and platinum sensitivity (p < 0.001) were independent predictors of improved survival in the PCS but not in the NAC group. Patients in the NAC group had significantly worse overall survival than those in the PCS group (31.6 v. 61.3 mo, p < 0.001).
Conclusion: Women with advanced HGSC who underwent PCS had better survival than those who underwent interval NAC, regardless of the number of cycles of neoadjuvant therapy. Optimal cytoreduction did not provide a survival advantage in the NAC group.
Background: La chirurgie et la chimiothérapie sont habituellement le traitement recommandé pour les carcinomes ovariens séreux bien différenciés de haut grade. Nous avons étudié le taux de survie de patientes ayant subi une chirurgie initiale ou d'intervalle à divers moments après une chimiothérapie néoadjuvante et l'avons comparé avec celui de patientes ayant subi une chirurgie de réduction tumorale initiale et une chimiothérapie adjuvante.
Methods: Cette étude de cohorte rétrospective a été menée auprès de patientes présentant un carcinome de stade III ou IV. Les données cliniques ont été tirées de leur dossier médical. Les patientes ont été séparées en 2 groupes : le premier était formé des patientes ayant subi une chimiothérapie néoadjuvante et une chirurgie de réduction tumorale d'intervalle (groupe NAC), et le deuxième de celles ayant subi une chirurgie de réduction tumorale initiale et une chimiothérapie adjuvante (groupe PCS). On a stratifié les 2 groupes à l'aide de plusieurs variables cliniques.
Results: L'étude portait sur 334 patientes, soit 156 dans le groupe NAC et 178 dans le groupe PCS. Dans le groupe NAC, aucune corrélation n'a été observée entre le taux de survie des patientes et le temps écoulé entre la chimiothérapie néoadjuvante et la chirurgie de réduction tumorale d'intervalle (p < 0,001). La réduction tumorale optimale n'a eu aucune incidence sur le taux de survie global des patientes du groupe NAC (p < 0,001). La réduction tumorale optimale (p < 0,001) et la sensibilité au platine (p < 0,001) ont été ciblés comme étant 2 prédicateurs indépendants d'un taux de survie accru chez les patientes du groupe PCS, mais pas chez celles du groupe NAC. Le taux de survie des patientes du groupe NAC était beaucoup plus faible que celui des patientes du groupe PCS (31,6 mo contre 61,3 mo, p < 0,001).
Conclusion: Les femmes atteintes d'un carcinome ovarien séreux bien différencié de haut grade ayant subi une chirurgie de réduction tumorale initiale et une chimiothérapie adjuvante (PCS) ont affiché un taux de survie plus élevé que les patientes ayant subi une chimiothérapie néoadjuvante et une chirurgie de réduction tumorale d'intervalle (NAC), peu importe le nombre de cycles de chimiothérapie néoadjuvante. La réduction tumorale optimale n'a pas été associée à un taux de survie plus élevé chez ces dernières.
Figures

References
-
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. - PubMed
-
- Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–29. - PubMed
-
- Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–200. - PubMed
-
- du Bois A, Luck HJ, Meier W, et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst. 2003;95:1320–9. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
