LIGHT/IFN-γ triggers β cells apoptosis via NF-κB/Bcl2-dependent mitochondrial pathway
- PMID: 27241100
- PMCID: PMC5020636
- DOI: 10.1111/jcmm.12876
LIGHT/IFN-γ triggers β cells apoptosis via NF-κB/Bcl2-dependent mitochondrial pathway
Abstract
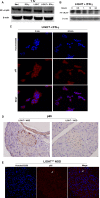
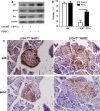
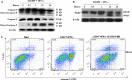
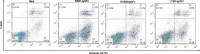
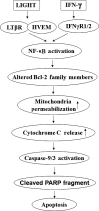
LIGHT recruits and activates naive T cells in the islets at the onset of diabetes. IFN-γ secreted by activated T lymphocytes is involved in beta cell apoptosis. However, whether LIGHT sensitizes IFNγ-induced beta cells destruction remains unclear. In this study, we used the murine beta cell line MIN6 and primary islet cells as models for investigating the underlying cellular mechanisms involved in LIGHT/IFNγ - induced pancreatic beta cell destruction. LIGHT and IFN-γ synergistically reduced MIN6 and primary islet cells viability; decreased cell viability was due to apoptosis, as demonstrated by a significant increase in Annexin V(+) cell percentage, detected by flow cytometry. In addition to marked increases in cytochrome c release and NF-κB activation, the combination of LIGHT and IFN-γ caused an obvious decrease in expression of the anti-apoptotic proteins Bcl-2 and Bcl-xL, but an increase in expression of the pro-apoptotic proteins Bak and Bax in MIN6 cells. Accordingly, LIGHT deficiency led to a decrease in NF-κB activation and Bak expression, and peri-insulitis in non-obese diabetes mice. Inhibition of NF-κB activation with the specific NF-κB inhibitor, PDTC (pyrrolidine dithiocarbamate), reversed Bcl-xL down-regulation and Bax up-regulation, and led to a significant increase in LIGHT- and IFN-γ-treated cell viability. Moreover, cleaved caspase-9, -3, and PARP (poly (ADP-ribose) polymerase) were observed after LIGHT and IFN-γ treatment. Pretreatment with caspase inhibitors remarkably attenuated LIGHT- and IFNγ-induced cell apoptosis. Taken together, our results indicate that LIGHT signalling pathway combined with IFN-γ induces beta cells apoptosis via an NF-κB/Bcl2-dependent mitochondrial pathway.
Keywords: LIGHT; NF-κB; apoptosis; mitochondrial stress; pancreatic beta cell.
© 2016 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures



Similar articles
-
Caspase-3 is involved in IFN-γ- and TNF-α-mediated MIN6 cells apoptosis via NF-κB/Bcl-2 pathway.Cell Biochem Biophys. 2013;67(3):1239-48. doi: 10.1007/s12013-013-9642-4. Cell Biochem Biophys. 2013. PMID: 23695786
-
STAT1-mediated down-regulation of Bcl-2 expression is involved in IFN-γ/TNF-α-induced apoptosis in NIT-1 cells.PLoS One. 2015 Mar 26;10(3):e0120921. doi: 10.1371/journal.pone.0120921. eCollection 2015. PLoS One. 2015. PMID: 25811609 Free PMC article.
-
Combination of all-trans retinoic acid and interferon-gamma suppressed PI3K/Akt survival pathway in glioblastoma T98G cells whereas NF-kappaB survival signaling in glioblastoma U87MG cells for induction of apoptosis.Neurochem Res. 2007 Dec;32(12):2194-202. doi: 10.1007/s11064-007-9417-7. Epub 2007 Jul 7. Neurochem Res. 2007. PMID: 17616812
-
Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities.Diabetes. 2005 Dec;54 Suppl 2:S97-107. doi: 10.2337/diabetes.54.suppl_2.s97. Diabetes. 2005. PMID: 16306347 Review.
-
Ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid induces apoptosis of human malignant cancer cells.Curr Drug Targets. 2012 Dec;13(14):1730-7. doi: 10.2174/138945012804545623. Curr Drug Targets. 2012. PMID: 23140284 Review.
Cited by
-
Gamma-tocopherol ameliorates hyperglycemia-induced hepatic inflammation associated with NLRP3 inflammasome in alloxan-induced diabetic mice.Nutr Res Pract. 2019 Oct;13(5):377-383. doi: 10.4162/nrp.2019.13.5.377. Epub 2019 Jul 10. Nutr Res Pract. 2019. PMID: 31583056 Free PMC article.
-
LIGHT deficiency attenuates acute kidney disease development in an in vivo experimental renal ischemia and reperfusion injury model.Cell Death Discov. 2022 Sep 26;8(1):399. doi: 10.1038/s41420-022-01188-x. Cell Death Discov. 2022. PMID: 36163116 Free PMC article.
-
Novel therapeutic strategies and perspectives for pancreatic cancer: Autophagy and apoptosis are key mechanisms to fight pancreatic cancer.Med Oncol. 2021 May 21;38(6):74. doi: 10.1007/s12032-021-01522-w. Med Oncol. 2021. PMID: 34019188 Review.
-
Endoplasmic reticulum stress and destruction of pancreatic β cells in type 1 diabetes.Chin Med J (Engl). 2020 Jan 5;133(1):68-73. doi: 10.1097/CM9.0000000000000583. Chin Med J (Engl). 2020. PMID: 31923106 Free PMC article. Review.
-
TNFSF14-HVEM/LTβR Exacerbates Keratinocyte Abnormalities and IMQ-Induced Psoriatic Skin Inflammation via Activating NF-κB/TWIST1 Signalling Pathway.J Cell Mol Med. 2025 Aug;29(15):e70774. doi: 10.1111/jcmm.70774. J Cell Mol Med. 2025. PMID: 40768619 Free PMC article.
References
-
- Kim HS, Lee MS. Role of innate immunity in triggering and tuning of autoimmune diabetes. Curr Mol Med. 2009; 9: 30–44. - PubMed
-
- Cao ZH, Yin WD, Zheng QY, et al Caspase‐3 is involved in IFN‐γ‐ and TNF‐α‐mediated MIN6 cells apoptosis via NF‐κB/Bcl‐2 pathway. Cell Biochem Biophys. 2013; 67: 1239–48. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

