Electromyographic evidence in support of a knock-in mouse model of DYT1 Dystonia
- PMID: 27241685
- PMCID: PMC5115930
- DOI: 10.1002/mds.26677
Electromyographic evidence in support of a knock-in mouse model of DYT1 Dystonia
Abstract
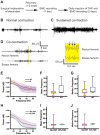
Introduction: DYT1 dystonia is an autosomal-dominant movement disorder characterized by abnormal, often repetitive, movements and postures. Its hallmark feature is sustained or intermittent contractions of muscles involving co-contractions of antagonist muscle pairs. The symptoms are relieved with the anticholinergic drug trihexyphenidyl. The primary mutation is a trinucleotide deletion (ΔGAG) in DYT1/TOR1A, which codes for torsinA. Previous studies showed that (1) heterozygous Dyt1 ΔGAG knock-in mice, which have an analogous mutation in the endogenous gene, exhibit motor deficits and altered corticostriatal synaptic plasticity in the brain and (2) these deficits can be rescued by trihexyphenidyl. However, brain imaging studies suggest that the Dyt1 knock-in mouse models nonmanifesting mutation carriers of DYT1 dystonia. The aim of this work was to examine the hallmark features of DYT1 dystonia in the Dyt1 knock-in mice by analyzing muscular activities.
Methods: Wireless telemetry devices with biopotential channels were implanted to the bicep and the rectus femori muscles in Dyt1 knock-in mice, and muscular activities were recorded before and after trihexyphenidyl administration.
Results: (1) Consistent with DYT1 dystonia patients, Dyt1 knock-in mice showed sustained contractions and co-contractions of the antagonistic bicep femoris and rectus femoris. (2) The abnormal muscle contractions were normalized by trihexyphenidyl.
Conclusion: The results suggest that the motor deficits in Dyt1 knock-in mice are likely produced by abnormal muscle contractions, and Dyt1 knock-in mice can potentially be used as a manifesting disease model to study pathophysiology and develop novel therapeutics. © 2016 International Parkinson and Movement Disorder Society.
Keywords: DYT1; anticholinergic; dystonia; electromyography; muscle contraction.
© 2016 International Parkinson and Movement Disorder Society.
Figures
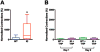

Comment on
-
Time to redefine PD? Introductory statement of the MDS Task Force on the definition of Parkinson's disease.Mov Disord. 2014 Apr;29(4):454-62. doi: 10.1002/mds.25844. Epub 2014 Mar 11. Mov Disord. 2014. PMID: 24619848 Free PMC article. Review.
References
-
- Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, Standaert DG. The pathophysiological basis of dystonias. Nat Rev Neurosci. 2008;9(3):222–234. - PubMed
-
- Ozelius LJ, Hewett JW, Page CE, et al. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nat Genet. 1997;17(1):40–48. - PubMed
-
- Caldwell GA, Cao S, Sexton EG, Gelwix CC, Bevel JP, Caldwell KA. Suppression of polyglutamine-induced protein aggregation in Caenorhabditis elegans by torsin proteins. Hum Mol Genet. 2003;12(3):307–319. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

