The exceptionally high reactivity of Cys 621 is critical for electrophilic activation of the sensory nerve ion channel TRPA1
- PMID: 27241698
- PMCID: PMC4886278
- DOI: 10.1085/jgp.201611581
The exceptionally high reactivity of Cys 621 is critical for electrophilic activation of the sensory nerve ion channel TRPA1
Abstract
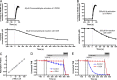
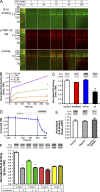
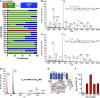
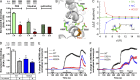
Activation of the sensory nerve ion channel TRPA1 by electrophiles is the key mechanism that initiates nociceptive signaling, and leads to defensive reflexes and avoidance behaviors, during oxidative stress in mammals. TRPA1 is rapidly activated by subtoxic levels of electrophiles, but it is unclear how TRPA1 outcompetes cellular antioxidants that protect cytosolic proteins from electrophiles. Here, using physiologically relevant exposures, we demonstrate that electrophiles react with cysteine residues on mammalian TRPA1 at rates that exceed the reactivity of typical cysteines by 6,000-fold and that also exceed the reactivity of antioxidant enzymes. We show that TRPA1 possesses a complex reactive cysteine profile in which C621 is necessary for electrophile-induced binding and activation. Modeling of deprotonation energies suggests that K620 contributes to C621 reactivity and mutation of K620 alone greatly reduces the effect of electrophiles on TRPA1. Nevertheless, binding of electrophiles to C621 is not sufficient for activation, which also depends on the function of another reactive cysteine (C665). Together, our results demonstrate that TRPA1 acts as an effective electrophilic sensor because of the exceptionally high reactivity of C621.
© 2016 Bahia et al.
Figures


References
-
- Becke A.D. 1993. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98:5648–5652. 10.1063/1.464913 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

