Measurement of protein size in concentrated solutions by small angle X-ray scattering
- PMID: 27241796
- PMCID: PMC4972196
- DOI: 10.1002/pro.2957
Measurement of protein size in concentrated solutions by small angle X-ray scattering
Abstract
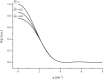
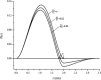
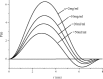
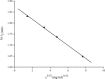
By simulations on the distance distribution function (DDF) derived from small angle X-ray scattering (SAXS) theoretical data of a dense monodisperse system, we found a quantitative mathematical correlation between the apparent size of a spherically symmetric (or nearly spherically symmetric) homogenous particle and the concentration of the solution. SAXS experiments on protein solutions of human hemoglobin and horse myoglobin validated the correlation. This gives a new method to determine, from the SAXS DDF, the size of spherically symmetric (or nearly spherically symmetric) particles of a dense monodisperse system, specifically for protein solutions with interference effects.
Keywords: distance distribution function; inter-particle interference; protein size; small angle X-ray scattering.
© 2016 The Protein Society.
Figures



Similar articles
-
Weak self-interactions of globular proteins studied by small-angle X-ray scattering and structure-based modeling.J Phys Chem B. 2014 Aug 28;118(34):10111-9. doi: 10.1021/jp505809v. Epub 2014 Aug 13. J Phys Chem B. 2014. PMID: 25117055
-
Atomic-resolution structural information from scattering experiments on macromolecules in solution.Phys Rev E Stat Nonlin Soft Matter Phys. 2013 May;87(5):052712. doi: 10.1103/PhysRevE.87.052712. Epub 2013 May 20. Phys Rev E Stat Nonlin Soft Matter Phys. 2013. PMID: 23767571
-
High-resolution wide-angle X-ray scattering of protein solutions: effect of beam dose on protein integrity.J Synchrotron Radiat. 2003 Sep 1;10(Pt 5):398-404. doi: 10.1107/s0909049503016583. Epub 2003 Aug 28. J Synchrotron Radiat. 2003. PMID: 12944630
-
A practical guide to small angle X-ray scattering (SAXS) of flexible and intrinsically disordered proteins.FEBS Lett. 2015 Sep 14;589(19 Pt A):2570-7. doi: 10.1016/j.febslet.2015.08.027. Epub 2015 Aug 29. FEBS Lett. 2015. PMID: 26320411 Review.
-
Structural characterization of proteins and complexes using small-angle X-ray solution scattering.J Struct Biol. 2010 Oct;172(1):128-41. doi: 10.1016/j.jsb.2010.06.012. Epub 2010 Jun 15. J Struct Biol. 2010. PMID: 20558299 Review.
Cited by
-
SAXS structure of homodimeric oxyHemoglobin III from bivalve Lucina pectinata.Biopolymers. 2021 Jun;112(6):e23427. doi: 10.1002/bip.23427. Epub 2021 Apr 1. Biopolymers. 2021. PMID: 33792032 Free PMC article.
-
Nonclassical Crystallization and Core-Shell Structure Formation of Ibuprofen from Binary Solvent Solutions.Cryst Growth Des. 2023 Jan 4;23(1):236-245. doi: 10.1021/acs.cgd.2c00971. Epub 2022 Dec 21. Cryst Growth Des. 2023. PMID: 36624777 Free PMC article.
References
-
- Glatter O, Kratky O (1982) Small angle X‐ray scattering. New York: Academic Press.
-
- Feigin LA, Svergun DI (1987) Structure analysis by small‐angle X‐ray and neutron scattering. New York: Plenum Press.
-
- Koch MHJ, Vachette P, Svergun DI (2003) Small‐angle scattering: a view on the properties, structures and structural changes of biological macromolecules in solution. Quart Rev Biophys 36:147–227. - PubMed
-
- Svergun DI, Koch MHJ, Timmins PA, May RP (2013) Small angle X‐ray and neutron scattering from solutions of biological macromolecules. Oxford University Press, USA.
-
- Choi MC, Raviv U, Li Y, Miller HP, Needleman DJ, Kim MW, Wilson L, Feinstein SC, Safinya CR (2011) Synchrotron small angle X‐ray scattering quantitatively detects angstrom level changes in the average radius of taxol‐stabilized microtubules decorated with the microtubule‐associated‐protein tau. J Phys Confer Ser 272:012001.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

