Measurement of protein size in concentrated solutions by small angle X-ray scattering
- PMID: 27241796
- PMCID: PMC4972196
- DOI: 10.1002/pro.2957
Measurement of protein size in concentrated solutions by small angle X-ray scattering
Abstract
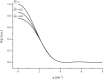
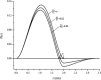
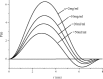
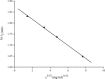
By simulations on the distance distribution function (DDF) derived from small angle X-ray scattering (SAXS) theoretical data of a dense monodisperse system, we found a quantitative mathematical correlation between the apparent size of a spherically symmetric (or nearly spherically symmetric) homogenous particle and the concentration of the solution. SAXS experiments on protein solutions of human hemoglobin and horse myoglobin validated the correlation. This gives a new method to determine, from the SAXS DDF, the size of spherically symmetric (or nearly spherically symmetric) particles of a dense monodisperse system, specifically for protein solutions with interference effects.
Keywords: distance distribution function; inter-particle interference; protein size; small angle X-ray scattering.
© 2016 The Protein Society.
Figures



References
-
- Glatter O, Kratky O (1982) Small angle X‐ray scattering. New York: Academic Press.
-
- Feigin LA, Svergun DI (1987) Structure analysis by small‐angle X‐ray and neutron scattering. New York: Plenum Press.
-
- Koch MHJ, Vachette P, Svergun DI (2003) Small‐angle scattering: a view on the properties, structures and structural changes of biological macromolecules in solution. Quart Rev Biophys 36:147–227. - PubMed
-
- Svergun DI, Koch MHJ, Timmins PA, May RP (2013) Small angle X‐ray and neutron scattering from solutions of biological macromolecules. Oxford University Press, USA.
-
- Choi MC, Raviv U, Li Y, Miller HP, Needleman DJ, Kim MW, Wilson L, Feinstein SC, Safinya CR (2011) Synchrotron small angle X‐ray scattering quantitatively detects angstrom level changes in the average radius of taxol‐stabilized microtubules decorated with the microtubule‐associated‐protein tau. J Phys Confer Ser 272:012001.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

