Postnatal telomere dysfunction induces cardiomyocyte cell-cycle arrest through p21 activation
- PMID: 27241915
- PMCID: PMC4896054
- DOI: 10.1083/jcb.201510091
Postnatal telomere dysfunction induces cardiomyocyte cell-cycle arrest through p21 activation
Abstract
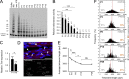
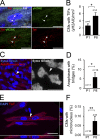
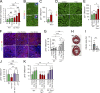
The molecular mechanisms that drive mammalian cardiomyocytes out of the cell cycle soon after birth remain largely unknown. Here, we identify telomere dysfunction as a critical physiological signal for cardiomyocyte cell-cycle arrest. We show that telomerase activity and cardiomyocyte telomere length decrease sharply in wild-type mouse hearts after birth, resulting in cardiomyocytes with dysfunctional telomeres and anaphase bridges and positive for the cell-cycle arrest protein p21. We further show that premature telomere dysfunction pushes cardiomyocytes out of the cell cycle. Cardiomyocytes from telomerase-deficient mice with dysfunctional telomeres (G3 Terc(-/-)) show precocious development of anaphase-bridge formation, p21 up-regulation, and binucleation. In line with these findings, the cardiomyocyte proliferative response after cardiac injury was lost in G3 Terc(-/-) newborns but rescued in G3 Terc(-/-)/p21(-/-) mice. These results reveal telomere dysfunction as a crucial signal for cardiomyocyte cell-cycle arrest after birth and suggest interventions to augment the regeneration capacity of mammalian hearts.
© 2016 Aix et al.
Figures




References
-
- Bär C., Bernardes de Jesus B., Serrano R., Tejera A., Ayuso E., Jimenez V., Formentini I., Bobadilla M., Mizrahi J., de Martino A., et al. . 2014. Telomerase expression confers cardioprotection in the adult mouse heart after acute myocardial infarction. Nat. Commun. 5:5863 10.1038/ncomms6863 - DOI - PMC - PubMed
-
- Bednarek D., González-Rosa J.M., Guzmán-Martínez G., Gutiérrez-Gutiérrez Ó., Aguado T., Sánchez-Ferrer C., Marques I.J., Galardi-Castilla M., de Diego I., Gómez M.J., et al. . 2015. Telomerase is essential for Zebrafish heart regeneration. Cell Reports. 12:1691–1703. 10.1016/j.celrep.2015.07.064 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

