Regulation of polar auxin transport by protein and lipid kinases
- PMID: 27242371
- PMCID: PMC4968656
- DOI: 10.1093/jxb/erw216
Regulation of polar auxin transport by protein and lipid kinases
Abstract
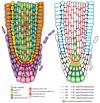
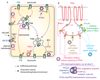
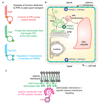
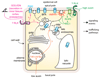
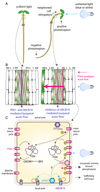
The directional transport of auxin, known as polar auxin transport (PAT), allows asymmetric distribution of this hormone in different cells and tissues. This system creates local auxin maxima, minima, and gradients that are instrumental in both organ initiation and shape determination. As such, PAT is crucial for all aspects of plant development but also for environmental interaction, notably in shaping plant architecture to its environment. Cell to cell auxin transport is mediated by a network of auxin carriers that are regulated at the transcriptional and post-translational levels. Here we review our current knowledge on some aspects of the 'non-genomic' regulation of auxin transport, placing an emphasis on how phosphorylation by protein and lipid kinases controls the polarity, intracellular trafficking, stability, and activity of auxin carriers. We describe the role of several AGC kinases, including PINOID, D6PK, and the blue light photoreceptor phot1, in phosphorylating auxin carriers from the PIN and ABCB families. We also highlight the function of some receptor-like kinases (RLKs) and two-component histidine kinase receptors in PAT, noting that there are probably RLKs involved in co-ordinating auxin distribution yet to be discovered. In addition, we describe the emerging role of phospholipid phosphorylation in polarity establishment and intracellular trafficking of PIN proteins. We outline these various phosphorylation mechanisms in the context of primary and lateral root development, leaf cell shape acquisition, as well as root gravitropism and shoot phototropism.
Keywords: Arabidopsis; auxin; cytokinin; endocytosis; gravitropism; intracellular trafficking; kinase; phosphoinositide; phototropism; polarity; receptor-like kinase.; root.
© The Author 2016. Published by Oxford University Press on behalf of the Society for Experimental Biology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures



References
-
- Abas L, Benjamins R, Malenica N, Paciorek T, Wisniewska J, Moulinier-Anzola JC, Sieberer T, Friml J, Luschnig C. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nature Cell Biology. 2006;8:249–256. - PubMed
-
- Ballesteros I, Dominguez T, Sauer M, Paredes P, Duprat A, Rojo E, Sanmartin M, Sanchez-Serrano JJ. Specialized functions of the PP2A subfamily II catalytic subunits PP2A-C3 and PP2A-C4 in the distribution of auxin fluxes and development in Arabidopsis. The Plant Journal. 2013;73:862–872. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

