Abnormal Changes of Brain Cortical Anatomy and the Association with Plasma MicroRNA107 Level in Amnestic Mild Cognitive Impairment
- PMID: 27242521
- PMCID: PMC4870937
- DOI: 10.3389/fnagi.2016.00112
Abnormal Changes of Brain Cortical Anatomy and the Association with Plasma MicroRNA107 Level in Amnestic Mild Cognitive Impairment
Abstract
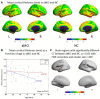
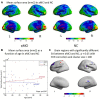
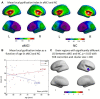
MicroRNA107 (Mir107) has been thought to relate to the brain structure phenotype of Alzheimer's disease. In this study, we evaluated the cortical anatomy in amnestic mild cognitive impairment (aMCI) and the relation between cortical anatomy and plasma levels of Mir107 and beta-site amyloid precursor protein (APP) cleaving enzyme 1 (BACE1). Twenty aMCI (20 aMCI) and 24 cognitively normal control (NC) subjects were recruited, and T1-weighted MR images were acquired. Cortical anatomical measurements, including cortical thickness (CT), surface area (SA), and local gyrification index (LGI), were assessed. Quantitative RT-PCR was used to examine plasma expression of Mir107, BACE1 mRNA. Thinner cortex was found in aMCI in areas associated with episodic memory and language, but with thicker cortex in other areas. SA decreased in aMCI in the areas associated with working memory and emotion. LGI showed a significant reduction in aMCI in the areas involved in language function. Changes in Mir107 and BACE1 messenger RNA plasma expression were correlated with changes in CT and SA. We found alterations in key left brain regions associated with memory, language, and emotion in aMCI that were significantly correlated with plasma expression of Mir107 and BACE1 mRNA. This combination study of brain anatomical alterations and gene information may shed lights on our understanding of the pathology of AD.
Clinical trial registration: http://www.ClinicalTrials.gov, identifier NCT01819545.
Keywords: Alzheimer’s disease; amnestic mild cognitive impairment; biological markers; genetics; surface-based morphometry.
Figures

References
-
- Albert M. S., DeKosky S. T., Dickson D., Dubois B., Feldman H. H., Fox N. C., et al. . (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 270–279. 10.1016/j.jalz.2011.03.008 - DOI - PMC - PubMed
-
- Casanova M. F., El-Baz A., Vanbogaert E., Narahari P., Switala A. (2010). A topographic study of minicolumnar core width by lamina comparison between autistic subjects and controls: possible minicolumnar disruption due to an anatomical element in-common to multiple laminae. Brain Pathol. 20, 451–458. 10.1111/j.1750-3639.2009.00319.x - DOI - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

