β-Adrenoceptor-mediated Relaxation of Urinary Bladder Muscle in β2-Adrenoceptor Knockout Mice
- PMID: 27242525
- PMCID: PMC4860462
- DOI: 10.3389/fphar.2016.00118
β-Adrenoceptor-mediated Relaxation of Urinary Bladder Muscle in β2-Adrenoceptor Knockout Mice
Abstract
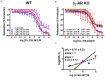
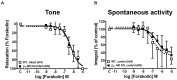
Background and objective: In order to characterize the β-adrenoceptor (AR) subtypes involved in agonist-stimulated relaxation of murine urinary bladder we studied the effects of (-)-isoprenaline and CL 316,243 on tonic contraction and spontaneous contractions in detrusor strips of wild-type (WT) and β2-AR knockout (β2-AR KO) mice.
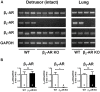
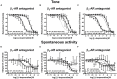
Materials and methods: Urinary bladders were isolated from male WT and β2-AR KO mice. β-AR subtype expression was determined with quantitative real-time PCR. Intact muscle strips pre-contracted with KCl (40 mM) were exposed to cumulatively increasing concentrations of (-)-isoprenaline or β3-AR agonist CL 316,243 in the presence and absence of the subtype-selective β-AR blockers CGP 20712A (β1-ARs), ICI 118,551 (β2-ARs), and L748,337 (β3-ARs).
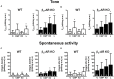
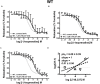
Results: Quantitative real-time PCR confirmed lack of β2-AR expression in bladder tissue from β2-AR KO mice. In isolated detrusor strips, pre-contraction with KCl increased basal tone and enhanced spontaneous activity significantly more in β2-AR KO than in WT. (-)-Isoprenaline relaxed tonic tension and attenuated spontaneous activity with similar potency, but the concentrations required were two orders of magnitude higher in β2-AR KO than WT. The concentration-response curves (CRCs) for relaxation were not affected by CGP 20712A (300 nM), but were shifted to the right by ICI 118,551 (50 nM) and L748,337 (10 μM). The -logEC50 values for (-)-isoprenaline in WT and β2-AR KO tissue were 7.98 and 6.00, respectively, suggesting a large receptor reserve of β2-AR. (-)-CL 316,243 relaxed detrusor and attenuated spontaneous contractions from WT and β2-AR KO mice with a potency corresponding to the drug's affinity for β3-AR. L743,337 shifted the CRCs to the right.
Conclusion: Our findings in β2-AR KO mice suggest that there is a large receptor reserve for β2-AR in WT mice so that this β-AR subtype will mediate relaxation of tone and attenuation of spontaneous activity under physiological conditions. Nevertheless, upon removal of this reserve, β3-AR can also mediate murine detrusor relaxation.
Keywords: CL 316,243; detrusor muscle; isoprenaline; mucosa; relaxation; β2-adrenoceptor knockout; β3-adrenoceptors.
Figures


Similar articles
-
β-Adrenoceptor-Mediated Relaxation of Carbachol-Pre-Contracted Mouse Detrusor.Urol Int. 2015;95(1):92-8. doi: 10.1159/000369075. Epub 2015 Jan 30. Urol Int. 2015. PMID: 25660359
-
Mucosa of murine detrusor impairs β2 -adrenoceptor-mediated relaxation.Neurourol Urodyn. 2015 Aug;34(6):592-7. doi: 10.1002/nau.22627. Epub 2014 May 12. Neurourol Urodyn. 2015. PMID: 24820256
-
Mucosa of human detrusor impairs contraction and β-adrenoceptor-mediated relaxation.BJU Int. 2013 Dec;112(8):1215-22. doi: 10.1111/bju.12267. Epub 2013 Aug 13. BJU Int. 2013. PMID: 23937341
-
The promise of beta3-adrenoceptor agonists to treat the overactive bladder.Urol Clin North Am. 2006 Nov;33(4):539-43, x. doi: 10.1016/j.ucl.2006.06.014. Urol Clin North Am. 2006. PMID: 17011390 Review.
-
Beta3-adrenoceptors in human detrusor muscle.Urology. 2002 May;59(5 Suppl 1):25-9. doi: 10.1016/s0090-4295(01)01635-1. Urology. 2002. PMID: 12007519 Review.
Cited by
-
Loss of resilience contributes to detrusor underactivity in advanced age.Biogerontology. 2023 Apr;24(2):163-181. doi: 10.1007/s10522-022-10005-y. Epub 2023 Jan 10. Biogerontology. 2023. PMID: 36626035 Free PMC article.
-
Therapeutic effects and modulatory mechanism of Alpiniae oxyphyllae Fructus in chronic intermittent hypoxia induced enuresis in rats.Sleep Breath. 2020 Mar;24(1):329-337. doi: 10.1007/s11325-019-01983-4. Epub 2020 Jan 2. Sleep Breath. 2020. PMID: 31898190
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

