Therapeutic Approaches to Genetic Ion Channelopathies and Perspectives in Drug Discovery
- PMID: 27242528
- PMCID: PMC4861771
- DOI: 10.3389/fphar.2016.00121
Therapeutic Approaches to Genetic Ion Channelopathies and Perspectives in Drug Discovery
Abstract
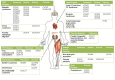
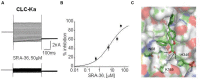
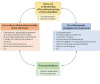
In the human genome more than 400 genes encode ion channels, which are transmembrane proteins mediating ion fluxes across membranes. Being expressed in all cell types, they are involved in almost all physiological processes, including sense perception, neurotransmission, muscle contraction, secretion, immune response, cell proliferation, and differentiation. Due to the widespread tissue distribution of ion channels and their physiological functions, mutations in genes encoding ion channel subunits, or their interacting proteins, are responsible for inherited ion channelopathies. These diseases can range from common to very rare disorders and their severity can be mild, disabling, or life-threatening. In spite of this, ion channels are the primary target of only about 5% of the marketed drugs suggesting their potential in drug discovery. The current review summarizes the therapeutic management of the principal ion channelopathies of central and peripheral nervous system, heart, kidney, bone, skeletal muscle and pancreas, resulting from mutations in calcium, sodium, potassium, and chloride ion channels. For most channelopathies the therapy is mainly empirical and symptomatic, often limited by lack of efficacy and tolerability for a significant number of patients. Other channelopathies can exploit ion channel targeted drugs, such as marketed sodium channel blockers. Developing new and more specific therapeutic approaches is therefore required. To this aim, a major advancement in the pharmacotherapy of channelopathies has been the discovery that ion channel mutations lead to change in biophysics that can in turn specifically modify the sensitivity to drugs: this opens the way to a pharmacogenetics strategy, allowing the development of a personalized therapy with increased efficacy and reduced side effects. In addition, the identification of disease modifiers in ion channelopathies appears an alternative strategy to discover novel druggable targets.
Keywords: channelopathies; drug discovery and development; genetics; ion channels pharmacology; physiopathology.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

