The Inositide Signaling Pathway As a Target for Treating Gastric Cancer and Colorectal Cancer
- PMID: 27242542
- PMCID: PMC4861839
- DOI: 10.3389/fphys.2016.00168
The Inositide Signaling Pathway As a Target for Treating Gastric Cancer and Colorectal Cancer
Abstract
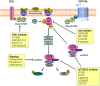
Gastric cancer and colorectal cancer are the leading cause of cancer mortality and have a dismal prognosis. The introduction of biological agents to treat these cancers has resulted in improved outcomes, and combination chemotherapy with targeted agents and conventional chemotherapeutic agents is regarded as standard therapy. Additional newly clarified mechanisms of oncogenesis and resistance to targeted agents require the development of new biologic agents. Aberrant activation of the inositide signaling pathway by a loss of function PTEN mutation or gain of function mutation/amplification of PIK3CA is an oncogenic mechanism in gastric cancer and colorectal cancer. Clinical trials with biologic agents that target the inositide signaling pathway are being performed to further improve treatment outcomes of patients with advanced gastric cancer and metastatic colorectal cancer (CRC). In this review we summarize the inositide signaling pathway, the targeted agents that inhibit abnormal activation of this signaling pathway and the clinical trials currently being performed in patients with advanced or metastatic gastric cancer and metastatic CRC using these targeted agents.
Keywords: Akt; PI3K; colorectal cancer; gastric cancer; mTOR; phosphoinositide; targeted therapy.
Figures
References
-
- Berndt N., Yang H., Trinczek B., Betzi S., Zhang Z., Wu B., et al. (2010). The Akt activation inhibitor TCN-P inhibits Akt phosphorylation by binding to the PH domain of Akt and blocking its recruitment to the plasma membrane. Cell Death Differ. 17, 1795–1804. 10.1038/cdd.2010.63 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

