Interactive Effects of Dorsomedial Hypothalamic Nucleus and Time-Restricted Feeding on Fractal Motor Activity Regulation
- PMID: 27242548
- PMCID: PMC4870237
- DOI: 10.3389/fphys.2016.00174
Interactive Effects of Dorsomedial Hypothalamic Nucleus and Time-Restricted Feeding on Fractal Motor Activity Regulation
Abstract
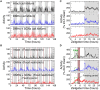
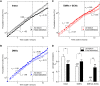
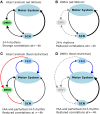
One evolutionary adaptation in motor activity control of animals is the anticipation of food that drives foraging under natural conditions and is mimicked in laboratory with daily scheduled food availability. Food anticipation is characterized by increased activity a few hours before the feeding period. Here we report that 2-h food availability during the normal inactive phase of rats not only increases activity levels before the feeding period but also alters the temporal organization of motor activity fluctuations over a wide range of time scales from minutes up to 24 h. We demonstrate this multiscale alteration by assessing fractal patterns in motor activity fluctuations-similar fluctuation structure at different time scales-that are robust in intact animals with ad libitum food access but are disrupted under food restriction. In addition, we show that fractal activity patterns in rats with ad libitum food access are also perturbed by lesion of the dorsomedial hypothalamic (DMH)-a neural node that is involved in food anticipatory behavior. Instead of further disrupting fractal regulation, food restriction restores the disrupted fractal patterns in these animals after the DMH lesion despite the persistence of the 24-h rhythms. This compensatory effect of food restriction is more clearly pronounced in the same animals after the additional lesion of the suprachiasmatic nucleus (SCN)-the central master clock in the circadian system that generates and orchestrates circadian rhythms in behavior and physiological functions in synchrony with day-night cycles. Moreover, all observed influences of food restriction persist even when data during the food anticipatory and feeding period are excluded. These results indicate that food restriction impacts dynamics of motor activity at different time scales across the entire circadian/daily cycle, which is likely caused by the competition between the food-induced time cue and the light-entrained circadian rhythm of the SCN. The differential impacts of food restriction on fractal activity control in intact and DMH-lesioned animals suggest that the DMH plays a crucial role in integrating these different time cues to the circadian network for multiscale regulation of motor activity.
Keywords: circadian rhythm; dorsomedial hypothalamic nucleus; food anticipation; fractal regulation; motor activity; suprachiasmatic nucleus.
Figures
Similar articles
-
Fractal Regulation in Temporal Activity Fluctuations: A Biomarker for Circadian Control and Beyond.JSM Biomark. 2017;3(1):1008. Epub 2017 Jan 17. JSM Biomark. 2017. PMID: 28553673 Free PMC article.
-
Circadian rhythms of PERIOD1 expression in the dorsomedial hypothalamic nucleus in the absence of entrained food-anticipatory activity rhythms in rats.Eur J Neurosci. 2009 Jun;29(11):2217-22. doi: 10.1111/j.1460-9568.2009.06766.x. Epub 2009 May 21. Eur J Neurosci. 2009. PMID: 19490091
-
Evidence for time-of-day dependent effect of neurotoxic dorsomedial hypothalamic lesions on food anticipatory circadian rhythms in rats.PLoS One. 2011;6(9):e24187. doi: 10.1371/journal.pone.0024187. Epub 2011 Sep 2. PLoS One. 2011. PMID: 21912674 Free PMC article.
-
Neurobiology of food anticipatory circadian rhythms.Physiol Behav. 2011 Sep 26;104(4):535-45. doi: 10.1016/j.physbeh.2011.04.015. Epub 2011 Apr 20. Physiol Behav. 2011. PMID: 21527266 Review.
-
[Circadian regulation of sleep-wake cycles and food anticipation].Brain Nerve. 2012 Jun;64(6):647-56. Brain Nerve. 2012. PMID: 22647472 Review. Japanese.
Cited by
-
Progression of Dementia Assessed by Temporal Correlations of Physical Activity: Results From a 3.5-Year, Longitudinal Randomized Controlled Trial.Sci Rep. 2016 Jun 13;6:27742. doi: 10.1038/srep27742. Sci Rep. 2016. PMID: 27292543 Free PMC article. Clinical Trial.
-
Reduced Tolerance to Night Shift in Chronic Shift Workers: Insight From Fractal Regulation.Sleep. 2017 Jul 1;40(7):zsx092. doi: 10.1093/sleep/zsx092. Sleep. 2017. PMID: 28838129 Free PMC article.
-
Dietary restriction modulates ultradian rhythms and autocorrelation properties in mice behavior.Commun Biol. 2024 Mar 9;7(1):303. doi: 10.1038/s42003-024-05991-3. Commun Biol. 2024. PMID: 38461321 Free PMC article.
-
Bombesin receptor subtype-3-expressing neurons regulate energy homeostasis through a novel neuronal pathway in the hypothalamus.Brain Behav. 2017 Dec 15;8(1):e00881. doi: 10.1002/brb3.881. eCollection 2018 Jan. Brain Behav. 2017. PMID: 29568682 Free PMC article.
-
Carnitine Acetyltransferase in AgRP Neurons Is Required for the Homeostatic Adaptation to Restricted Feeding in Male Mice.Endocrinology. 2018 Jun 1;159(6):2473-2483. doi: 10.1210/en.2018-00131. Endocrinology. 2018. PMID: 29697769 Free PMC article.
References
-
- Acosta-Galvan G., Yi C.-X., van der Vliet J., Jhamandas J. H., Panula P., Angeles-Castellanos M., et al. . (2011). Interaction between hypothalamic dorsomedial nucleus and the suprachiasmatic nucleus determines intensity of food anticipatory behavior. Proc. Natl. Acad. Sci. U.S.A. 108, 5813–5818. 10.1073/pnas.1015551108 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

