The Genetic Basis of Cognitive Impairment and Dementia in Parkinson's Disease
- PMID: 27242557
- PMCID: PMC4873499
- DOI: 10.3389/fpsyt.2016.00089
The Genetic Basis of Cognitive Impairment and Dementia in Parkinson's Disease
Abstract
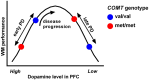
Cognitive dysfunction is a common feature of Parkinson's disease (PD) with mild cognitive impairment affecting around a quarter of patients in the early stages of their disease, and approximately half developing dementia by 10 years from diagnosis. However, the pattern of cognitive impairments and their speed of evolution vary markedly between individuals. While some of this variability may relate to extrinsic factors and comorbidities, inherited genetic heterogeneity is also known to play an important role. A number of common genetic variants have been identified, which contribute to cognitive function in PD, including variants in catechol-O-methyltransferase, microtubule-associated protein tau, and apolipoprotein E. Furthermore, rarer mutations in glucocerebrosidase and α-synuclein and are strongly associated with dementia risk in PD. This review explores the functional impact of these variants on cognition in PD and discusses how such genotype-phenotype associations provide a window into the mechanistic basis of cognitive heterogeneity in this disorder. This has consequent implications for the development of much more targeted therapeutic strategies for cognitive symptoms in PD.
Keywords: APOE; COMT; GBA; MAPT; Parkinson’s disease; cognition; dementia; genetics.
Figures
References
-
- Bohnen NI, Kaufer DI, Ivanco LS, Lopresti B, Koeppe RA, Davis JG, et al. Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease: an in vivo positron emission tomographic study. Arch Neurol (2003) 60:1745–8.10.1001/archneur.60.12.1745 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

