T-Type Calcium Channel: A Privileged Gate for Calcium Entry and Control of Adrenal Steroidogenesis
- PMID: 27242667
- PMCID: PMC4873500
- DOI: 10.3389/fendo.2016.00043
T-Type Calcium Channel: A Privileged Gate for Calcium Entry and Control of Adrenal Steroidogenesis
Abstract
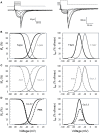
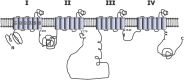
Intracellular calcium plays a crucial role in modulating a variety of functions such as muscle contraction, hormone secretion, gene expression, or cell growth. Calcium signaling has been however shown to be more complex than initially thought. Indeed, it is confined within cell microdomains, and different calcium channels are associated with different functions, as shown by various channelopathies. Sporadic mutations on voltage-operated L-type calcium channels in adrenal glomerulosa cells have been shown recently to be the second most prevalent genetic abnormalities present in human aldosterone-producing adenoma. The observed modification of the threshold of activation of the mutated channels not only provides an explanation for this gain of function but also reminds us on the importance of maintaining adequate electrophysiological characteristics to make channels able to exert specific cellular functions. Indeed, the contribution to steroid production of the various calcium channels expressed in adrenocortical cells is not equal, and the reason has been investigated for a long time. Given the very negative resting potential of these cells, and the small membrane depolarization induced by their physiological agonists, low threshold T-type calcium channels are particularly well suited for responding under these conditions and conveying calcium into the cell, at the right place for controlling steroidogenesis. In contrast, high threshold L-type channels are normally activated by much stronger cell depolarizations. The fact that dihydropyridine calcium antagonists, specific for L-type channels, are poorly efficient for reducing aldosterone secretion either in vivo or in vitro, strongly supports the view that these two types of channels differently affect steroid biosynthesis. Whether a similar analysis is transposable to fasciculata cells and cortisol secretion is one of the questions addressed in the present review. No similar mutations on L-type or T-type channels have been described yet to affect cortisol secretion or to be linked to the development of Cushing syndrome, but several evidences suggest that the function of T channels is also crucial in fasciculata cells. Putative molecular mechanisms and cellular structural organization making T channels a privileged entry for the "steroidogenic calcium" are also discussed.
Keywords: ACTH; T-type calcium channels; adrenal cortex; aldosterone; calcium signaling; cortisol; electrophysiology; steroidogenesis.
Figures

References
-
- Rossier MF. Function and differential expression of T-type calcium channels in various pathophysiological states. Recent Res Dev Biochem (2003) 4:13–29.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

