Metabolic Network Modeling of Microbial Interactions in Natural and Engineered Environmental Systems
- PMID: 27242701
- PMCID: PMC4870247
- DOI: 10.3389/fmicb.2016.00673
Metabolic Network Modeling of Microbial Interactions in Natural and Engineered Environmental Systems
Abstract
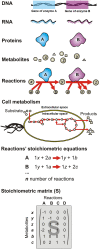
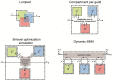
We review approaches to characterize metabolic interactions within microbial communities using Stoichiometric Metabolic Network (SMN) models for applications in environmental and industrial biotechnology. SMN models are computational tools used to evaluate the metabolic engineering potential of various organisms. They have successfully been applied to design and optimize the microbial production of antibiotics, alcohols and amino acids by single strains. To date however, such models have been rarely applied to analyze and control the metabolism of more complex microbial communities. This is largely attributed to the diversity of microbial community functions, metabolisms, and interactions. Here, we firstly review different types of microbial interaction and describe their relevance for natural and engineered environmental processes. Next, we provide a general description of the essential methods of the SMN modeling workflow including the steps of network reconstruction, simulation through Flux Balance Analysis (FBA), experimental data gathering, and model calibration. Then we broadly describe and compare four approaches to model microbial interactions using metabolic networks, i.e., (i) lumped networks, (ii) compartment per guild networks, (iii) bi-level optimization simulations, and (iv) dynamic-SMN methods. These approaches can be used to integrate and analyze diverse microbial physiology, ecology and molecular community data. All of them (except the lumped approach) are suitable for incorporating species abundance data but so far they have been used only to model simple communities of two to eight different species. Interactions based on substrate exchange and competition can be directly modeled using the above approaches. However, interactions based on metabolic feedbacks, such as product inhibition and synthropy require extensions to current models, incorporating gene regulation and compounding accumulation mechanisms. SMN models of microbial interactions can be used to analyze complex "omics" data and to infer and optimize metabolic processes. Thereby, SMN models are suitable to capitalize on advances in high-throughput molecular and metabolic data generation. SMN models are starting to be applied to describe microbial interactions during wastewater treatment, in-situ bioremediation, microalgae blooms methanogenic fermentation, and bioplastic production. Despite their current challenges, we envisage that SMN models have future potential for the design and development of novel growth media, biochemical pathways and synthetic microbial associations.
Keywords: environmental biotechnology; flux balance analysis; genome-scale metabolic model; metabolic network; microbial communities; process engineering; systems biology; wastewater treatment.
Figures


References
-
- Abubackar H. N., Veiga M. C., Kennes C. (2011). Biological conversion of carbon monoxide: rich syngas or waste gases to bioethanol. Biofuel Bioprod. Biorefin. 5, 93–114. 10.1002/bbb.256 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

