Dynamics of Actin Cables in Polarized Growth of the Filamentous Fungus Aspergillus nidulans
- PMID: 27242709
- PMCID: PMC4860496
- DOI: 10.3389/fmicb.2016.00682
Dynamics of Actin Cables in Polarized Growth of the Filamentous Fungus Aspergillus nidulans
Abstract
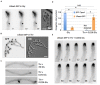
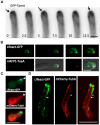
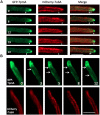
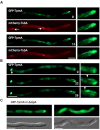
Highly polarized growth of filamentous fungi requires a continuous supply of proteins and lipids to the hyphal tip. This transport is managed by vesicle trafficking via the actin and microtubule cytoskeletons and their associated motor proteins. Particularly, actin cables originating from the hyphal tip are essential for hyphal growth. Although, specific marker proteins have been developed to visualize actin cables in filamentous fungi, the exact organization and dynamics of actin cables has remained elusive. Here, we observed actin cables using tropomyosin (TpmA) and Lifeact fused to fluorescent proteins in living Aspergillus nidulans hyphae and studied the dynamics and regulation. GFP tagged TpmA visualized dynamic actin cables formed from the hyphal tip with cycles of elongation and shrinkage. The elongation and shrinkage rates of actin cables were similar and approximately 0.6 μm/s. Comparison of actin markers revealed that high concentrations of Lifeact reduced actin dynamics. Simultaneous visualization of actin cables and microtubules suggests temporally and spatially coordinated polymerization and depolymerization between the two cytoskeletons. Our results provide new insights into the molecular mechanism of ordered polarized growth regulated by actin cables and microtubules.
Keywords: Aspergillus; actin; filamentous fungi; microtubule; polarity.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

