Cobalamin Protection against Oxidative Stress in the Acidophilic Iron-oxidizing Bacterium Leptospirillum Group II CF-1
- PMID: 27242761
- PMCID: PMC4876134
- DOI: 10.3389/fmicb.2016.00748
Cobalamin Protection against Oxidative Stress in the Acidophilic Iron-oxidizing Bacterium Leptospirillum Group II CF-1
Abstract
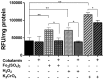
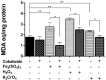
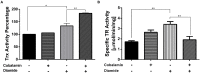
Members of the genus Leptospirillum are aerobic iron-oxidizing bacteria belonging to the phylum Nitrospira. They are important members of microbial communities that catalyze the biomining of sulfidic ores, thereby solubilizing metal ions. These microorganisms live under extremely acidic and metal-loaded environments and thus must tolerate high concentrations of reactive oxygen species (ROS). Cobalamin (vitamin B12) is a cobalt-containing tetrapyrrole cofactor involved in intramolecular rearrangement reactions and has recently been suggested to be an intracellular antioxidant. In this work, we investigated the effect of the exogenous addition of cobalamin on oxidative stress parameters in Leptospirillum group II strain CF-1. Our results revealed that the external supplementation of cobalamin reduces the levels of intracellular ROSs and the damage to biomolecules, and also stimulates the growth and survival of cells exposed to oxidative stress exerted by ferric ion, hydrogen peroxide, chromate and diamide. Furthermore, exposure of strain CF-1 to oxidative stress elicitors resulted in the transcriptional activation of the cbiA gene encoding CbiA of the cobalamin biosynthetic pathway. Altogether, these data suggest that cobalamin plays an important role in redox protection of Leptospirillum strain CF-1, supporting survival of this microorganism under extremely oxidative environmental conditions. Understanding the mechanisms underlying the protective effect of cobalamin against oxidative stress may help to develop strategies to make biomining processes more effective.
Keywords: Leptospirillum group II CF-1; bioleaching; cobalamin; heavy metals; oxidative stress; vitamin B12.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

