Elevated-CO2 Response of Stomata and Its Dependence on Environmental Factors
- PMID: 27242858
- PMCID: PMC4865672
- DOI: 10.3389/fpls.2016.00657
Elevated-CO2 Response of Stomata and Its Dependence on Environmental Factors
Abstract
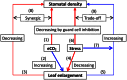
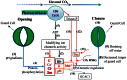
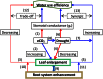
Stomata control the flow of gases between plants and the atmosphere. This review is centered on stomatal responses to elevated CO2 concentration and considers other key environmental factors and underlying mechanisms at multiple levels. First, an outline of general responses in stomatal conductance under elevated CO2 is presented. Second, stomatal density response, its development, and the trade-off with leaf growth under elevated CO2 conditions are depicted. Third, the molecular mechanism regulating guard cell movement at elevated CO2 is suggested. Finally, the interactive effects of elevated CO2 with other factors critical to stomatal behavior are reviewed. It may be useful to better understand how stomata respond to elevated CO2 levels while considering other key environmental factors and mechanisms, including molecular mechanism, biochemical processes, and ecophysiological regulation. This understanding may provide profound new insights into how plants cope with climate change.
Keywords: drought; elevated CO2; global warming; guard cell; mesophyll-driven signals; photosynthesis; regulation mechanism; stomatal behavior.
Figures
References
-
- Araújo W. L., Nunes-Nesi A., Osorio S., Usadel B., Fuentes D., Nagy R., et al. (2011). Antisense inhibition of the iron-sulphur subunit of succinate dehydrogenase enhances photosynthesis and growth in tomato via an organic acid–mediated effect on stomatal aperture. Plant Cell 23 600–627. 10.1105/tpc.110.081224 - DOI - PMC - PubMed
-
- Armstrong E., Valdes P., House J., Singarayer J. (2016). The role of CO2 and dynamic vegetation on the impact of temperate land use change in the HadCM3 coupled climate model. Earth Interact. 20 10.1175/EI-D-15-0036.1 - DOI
-
- Ashraf M., Harris P. J. C. (2013). Photosynthesis under stressful environments: an overview. Photosynthetica 51 163–190. 10.1007/s11099-013-0021-6 - DOI
-
- Assmann S. M. (1999). The cellular basis of guard cell sensing of rising CO2. Plant Cell Environ. 22 629–637. 10.1046/j.1365-3040.1999.00408.x - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

